MOF玻璃的多孔结构,对其在吸附、分离领域的应用至关重要。但是,在MOF晶体熔融过程中MOF的孔道发生坍塌、变形,使孔径变小和孔隙率降低。因此,选择孔径大、孔隙率高的可熔融MOF晶体作为MOF玻璃的前驱体,可将晶体结构中的多孔结构更多保留在玻璃中。SOD型的ZIF-8具有丰富的孔道结构[17,18],但是ZIF-8的化学组成为Zn(MIm)2,其中MIm即2-甲基咪唑使其熔点远高于分解温度,因此不能直接制备玻璃[14]。用溶剂协助配体交换合成方法可将ZIF-8配体换为Im而保留其拓扑结构,制得的MOF晶体称为SALEM-2[19]。SALEM-2的化学组成与ZIF-4类似,为可熔融淬火形成玻璃提供化学基础。同时,SALEM-2继承了其母体材料ZIF-8的多孔拓扑结构。因此,SALEM-2可能是制备多孔MOF玻璃的理想前驱体。鉴于此,本文将MOF晶体SALEM-2熔融淬火制备多孔玻璃agSALEM-2,研究淬火温度和速率等因素对agSALEM-2多孔结构的影响。
1 实验方法
1.1 实验用原料
咪唑(分析纯),正丁醇(分析纯),ZIF-8,氘代盐酸,氘代DMSO。
1.2 SALEM-2和 agSALEM-2的合成
将咪唑(2.94 mmol)置于25 mL微波瓶中,用超声将其溶解在正丁醇(20 mL)中,再将100 mg的ZIF-8晶体浸入其中。将微波瓶加盖后放置在温度为373 K的烘箱中反应7 d,得到SALEM-2[17]。
将SALEM-2置于同步热分析仪(TG-DSC)中,在Ar气氛下以5 K/min速率将其加热到淬火温度后在一定的降温速率淬火至室温,得到agSALEM-2。
1.3 结构和性能表征
用同步热分析仪(TG-DSC,STA 449C型)对样品进行热分析,温度区间为室温至1000 K,升温速率为5 K/min(Ar气氛)。用X射线粉末衍射仪(XRD,D8型)分析样品的晶体结构。用氘代盐酸和氘代DMSO的混合溶液将样品溶解后用核磁共振波谱仪(NMR,Bruker Avance III,500 MHz)分析其化学结构。用气体密度仪(AccuPyc II1340型)测试样品的密度,单次测试样品的用量约500 mg。用气体吸附仪(Autosorb iQ型)测试样品的等温N2吸脱附曲线,测试前在1.33 × 10-3 Pa和473.15 K条件下将样品脱气10 h。
2 结果和讨论
2.1 结构和熔融过程
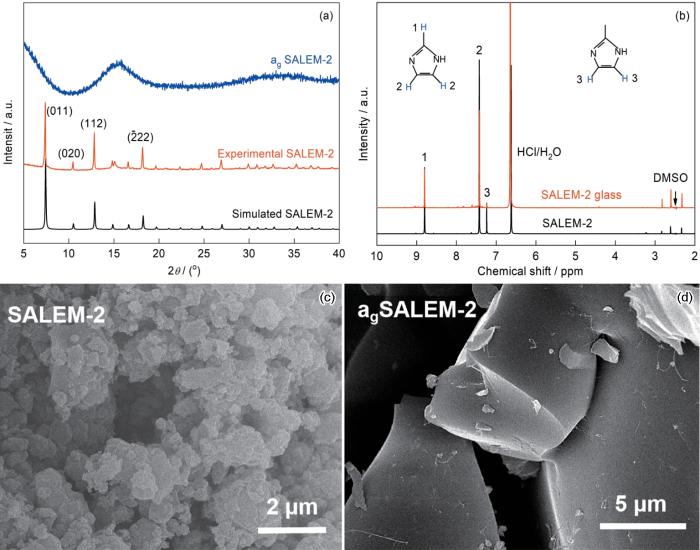
图1给出了SALEM-2和agSALEM-2的晶体结构和化学组成。实验中合成的晶体SALEM-2的衍射谱与文献中的模拟谱一致,表明其保留了多孔的SOD拓扑结构(图1a)。NMR结果表明,SALEM-2的有机配体中仍含有20%MIm,表明SALEM-2配体交换不完全,只有80%的有机配体交换为Im(图1b)。将SALEM-2熔融淬火制备的MOF玻璃agSALEM-2,其XRD谱(图1a)中所有衍射峰均消失,只剩下一个馒头峰。这表明,agSALEM-2的结构为长程无序,即无定形。从SALEM-2熔融前后的SEM照片(图1c,d)可见,SALEM-2的晶体形貌由颗粒转变为均匀致密的块状agSALEM-2,也表明熔融后其结构发生了变化。NMR谱(图1b)表明,agSALEM-2的有机配体中Im的含量从80%提高到95%,表明在MOF玻璃形成过程中发生了有机配体MIm的分解,从而使agSALEM-2中Im的含量比晶体中的高。
图1
图1
SALEM-2和agSALEM-2的晶体结构和化学组成
Fig.1
Characterization of crystal structure and chemical composition of SALEM-2 and agSALEM-2 (a) XRD results; (b) NMR results; (c) SEM image of SALEM crystals; (d) SEM image of agSALEM
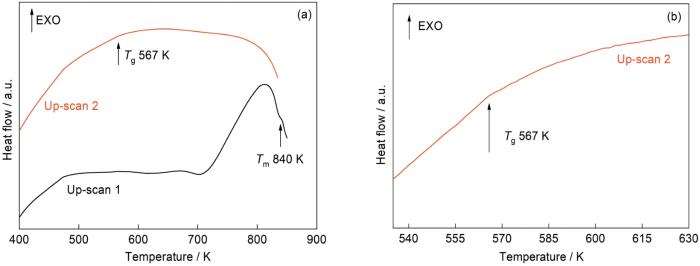
图2
图2
SALEM-2的熔融淬火过程
Fig.2
Characterization of SALEM-2 melt-quenching process (a) DSC results; (b) glass transition temperature Tg local DSC magnification
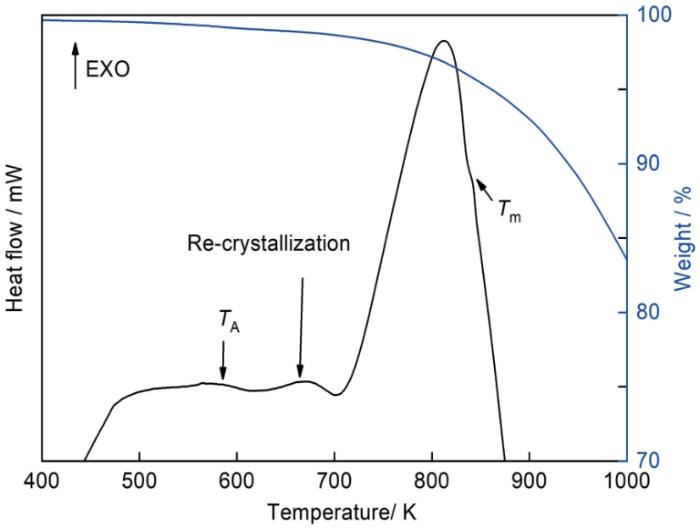
图3
图3
SALEM-2熔融过程中的TG-DSC曲线
Fig.3
TG-DSC results during the melting process of SALEM-2
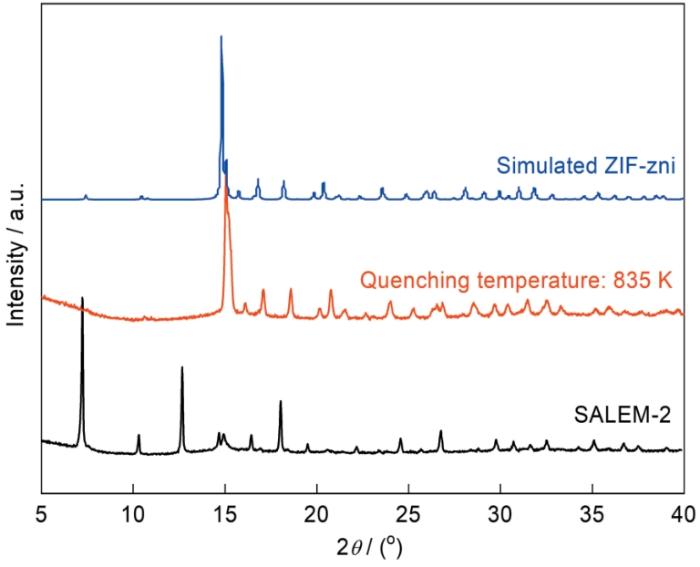
图4
图4
SALEM-2、淬火产物与模拟ZIF-zni的XRD谱
Fig.4
XRD patterns of SALEM-2, quenched product and simulated ZIF-zni
上述结果表明,MOF受热产生的热致无序、重结晶、熔化、分解等过程主要受其化学组成控制,与MOFs晶体的拓扑结构无关。
2.2 淬火温度和冷却速率的影响
淬火温度和冷却速率对agSALEM-2结构的影响,如图5所示。当淬火温度(835 K)低于熔点温度时,即使冷却速率较高(20 K/min),产物的谱中仍出现较强的衍射峰,表明该产物主要为ZIF-zni晶体。当淬火温度等于熔点温度(840 K)时,冷却速率对产品结构起着重要的影响。淬火温度为840 K、冷却速率为5 K/min时的产物主要为无定形态,而冷却速率为20 K/min时产物主要为ZIF-zni晶体。由此可见,淬火温度为熔点840 K时,较高的冷却速率使样品熔融不完全,不能得到完全无定形的玻璃。在高于熔点的温度(845 K)淬火,都能得到无定形agSALEM-2。因为淬火温度高于熔点,淬火前样品已经完全熔融。
图5
图5
在不同淬火温度和冷却速率条件下制备的agSALEM-2的XRD谱
Fig.5
XRD spectrum of the prepared products at different quenching temperatures and rates (a) 835 K, (b) 840 K, (c) 845 K (ISO 3 min denotes 845 K for 3 minutes)
使用气体密度仪测试了在845 K淬火、冷却速率不同得到的agSALEM-2的密度,结果如图6所示。可以看出,随着冷却速率的提高agSALEM-2的密度先降低而后提高。
图6
图6
淬火温度845 K时冷却速率对agSALEM-2密度的影响
Fig.6
Effects of quenching rates on density of agSALEM-2 formed at quenching temperature of 845 K
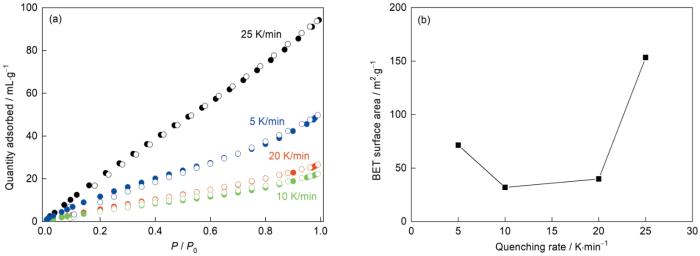
利用N2吸附实验研究冷却速率对agSALEM-2多孔性的影响,结果如图7所示。可以看出,随着冷却速率的降低,agSALEM-2的气体吸附量先下降后提高。随着冷却速率从25 K/min降低到10 K/min,agSALEM-2的BET比表面积从150 m2/g减小到约25 m2/g。而当冷却速率进一步降低到5 K/min时,agSALEM-2的BET比表面积又增大到75 m2/g。
图7
图7
淬火温度845 K时冷却速率不同的条件下agSALEM-2玻璃的吸附等温线和比表面积随冷却速率的变化
Fig.7
Results of N2 adsorption on agSALEM-2 glass at different quenching rates at 845 K (a) adsorption isotherms; (b) variation of specific surface area with quenching rate
上述结果表明,agSALEM-2的多孔结构是其结构中的MIm所致。在淬火过程中MIm阻碍了框架结构的重新形成,从而保留了液态下较大的孔结构,使N2得以进入。冷却速率越高其液态结构保留的越多,从而有更多的大孔吸附N2。同时,更多He不能进入的小孔也得以保留,因此测得的玻璃密度随着冷却速率的提高而提高。而当冷却速率为5 K/min,分解出的多孔碳较多,从而使N2吸附量增大和密度提高。熔化本质上是有机配体的解离和扩散,因此熔融后冷却速率对孔结构的影响与物质的迁移扩散有关,且熔融态驻留时间越长、分解程度越大,对孔结构的影响越显著。
文献报道SALEM-2晶体拓扑结构为SOD型,孔径为1.2 nm,孔容大小为0.75 cm3/g[21],实际测得等温N2吸附曲线类型为I型,BET比表面积为830 m2/g[15]。agSALEM-2的主要孔结构源于SALEM-2晶体孔结构的变形和坍塌,其等温N2吸附曲线为II型,出现吸附迟滞,BET比表面积为150 m2/g (冷却速率:25 K/min),表明agSALEM-2孔径变小且孔径通道更加曲折。同时,随着冷却速率的降低agSALEM-2比表面积出现由大变小再变大的趋势,表明其部分孔结构还是其配体分解所致。表1对比了agSALEM-2和与其结构类似的MOF玻璃(agZIF-4)及其它结构类型MOF玻璃的BET比表面积。如表1所示,agSALEM-2的BET比表面积远大于agZIF-4,表明Im的配体掺杂使agSALEM-2在一定程度上保留了ZIF-8的多孔拓扑结构,使其比表面积增大。与用熔融-淬火方法制备的其他结构类型MOF玻璃相比,agSALEM-2的BTE比表面积高出2~4个数量级,与用溶胶-凝胶方法制备的MOF玻璃处于同一数量级。这表明,本文实验制备的agSALEM-2其BET比表面积较高。
表1 MOF玻璃BET比表面积
Table 1
| MOF glass | Preparation methods | BET specific surface area [Ref.] |
|---|---|---|
| Ti-BPP | Sol-gel | 267 [22] |
| Ti-BPA | Sol-gel | 330 [22] |
| Ti-Fum | Sol-gel | 923 [23] |
| agZIF-4(Zn) | Melting-quenching | 2.921 [24] |
| agZIF-62 | Melting-quenching | 0.012 [24] |
| agTIF-4 | Melting-quenching | 0.094 [24] |
| agZn(Im)2 (GIS) | Melting-quenching | 0.450 [24] |
| agSALEM-2 | Melting-quenching | 150 [This work] |
因此,为了增加MOF玻璃的多孔结构和提高材料的多孔性,应该选择具有多孔拓扑结构的MOF晶体作为前驱体和在MOF的有机配体中“掺杂”尺寸较大的配体,利用空间位阻效应使MOF熔融状态下的多孔结构尽可能保留在玻璃中。
3 结论
SALEM-2熔融淬火后可生成无定形玻璃agSALEM-2。SALEM-2熔融前依次经历热致非晶化、重结晶和分解过程。重结晶的产物为ZIF-zni,在SALEM-2中残留的MIm分解导致缺陷,对熔融过程有显著的促进作用并使熔点降低。SALEM-2的熔融过程主要受其化学组成控制。在淬火过程中MIm阻碍框架结构的重新形成,从而部分保留其液态下的孔结构。淬火速率越高,液态下的多孔结构保留的越多。agSALEM-2的BET比表面积,最高可达150 m2/g。
参考文献
Introducing porosity into metal-organic framework glasses
[J].
Observation of indentation-induced shear bands in a metal-organic framework glass
[J].Metal-organic framework (MOF) glasses are a newly emerged family of melt-quenched glasses. Recently, several intriguing features, such as ultrahigh glass-forming ability and low liquid fragility, have been discovered in a number of zeolitic imidazolate frameworks (ZIFs) that are a subset of MOFs. However, the fracture behavior of ZIF glasses has not been explored. Here we report an observation of both cracking pattern and shear bands induced by indentation in a representative melt-quenched ZIF glass, that is, ZIF-62 glass (ZnImbIm). The shear banding in the ZIF glass is in strong contrast to the cracking behavior of other types of fully polymerized glasses, which do not exhibit any shear bands under indentation. We attribute this anomalous cracking behavior to the easy breakage of the coordinative bonds (Zn-N) in ZIF glasses, since these bonds are much weaker than the ionic and covalent bonds in network glasses.
Melt‐quenched hybrid glasses from metal-organic frameworks
[J].
Quantification of gas-accessible microporosity in metal-organic framework glasses
[J].Metal-organic framework (MOF) glasses are a new class of glass materials with immense potential for applications ranging from gas separation to optics and solid electrolytes. Due to the inherent difficulty to determine the atomistic structure of amorphous glasses, the intrinsic structural porosity of MOF glasses is only poorly understood. Here, we investigate the porosity features (pore size and pore limiting diameter) of a series of prototypical MOF glass formers from the family of zeolitic imidazolate frameworks (ZIFs) and their corresponding glasses. CO sorption at 195 K allows quantifying the microporosity of these materials in their crystalline and glassy states, also providing excess to the micropore volume and the apparent density of the ZIF glasses. Additional hydrocarbon sorption data together with X-ray total scattering experiments prove that the porosity features of the ZIF glasses depend on the types of organic linkers. This allows formulating design principles for a targeted tuning of the intrinsic microporosity of MOF glasses. These principles are counterintuitive and contrary to those established for crystalline MOFs but show similarities to strategies previously developed for porous polymers.© 2022. The Author(s).
Mechanical properties and processing techniques of bulk metal-organic framework glasses
[J].Melt quenched metal-organic framework (MOF) glasses define a new category of glass, distinct from metallic, organic, and inorganic glasses, owing to the dominant role of metal-ligand coordination bonding. The mechanical properties of glasses in general are important given their application in protective coatings and display technologies, though little is known about MOF glasses in this respect. The experimental elucidation of key properties such as their scratch resistance has been limited by the lack of processing methodologies capable of producing bulk glass samples. Here, nanoindentation was used to investigate the Young's modulus and hardness of four melt-quenched glasses formed from zeolitic imidazolate frameworks (ZIF): aZIF-4, aZIF-62, aZIF-76, and aZIF-76-mbIm. The creep resistance of the melt-quenched glasses was studied via strain-rate jump (SRJ) tests and through constant load and hold (CLH) indentation creep experiments. Values for the strain-rate sensitivity were found to be close to those for other glassy polymers and Se-rich GeSe chalcogenide glasses. Vacuum hot-pressing of aZIF-62 resulted in an inhomogeneous bulk sample containing the glass and amorphous non-melt-quenched aZIF-62. Remelting and annealing, however, resulted in the fabrication of a transparent, bubble-free bulk specimen, which allowed the first scratch testing experiments to be performed on an MOF glass.
Liquid metal-organic frameworks
[J].Metal-organic frameworks (MOFs) are a family of chemically diverse materials, with applications in a wide range of fields, covering engineering, physics, chemistry, biology and medicine. Until recently, research has focused almost entirely on crystalline structures, yet now a clear trend is emerging, shifting the emphasis onto disordered states, including 'defective by design' crystals, as well as amorphous phases such as glasses and gels. Here we introduce a strongly associated MOF liquid, obtained by melting a zeolitic imidazolate framework. We combine in situ variable temperature X-ray, ex situ neutron pair distribution function experiments, and first-principles molecular dynamics simulations to study the melting phenomenon and the nature of the liquid obtained. We demonstrate from structural, dynamical, and thermodynamical information that the chemical configuration, coordinative bonding, and porosity of the parent crystalline framework survive upon formation of the MOF liquid.
Metal-organic frameworks (MOFs) beyond crystallinity: amorphous MOFs, MOF liquids and MOF glasses
[J].
A MOF glass membrane for gas separation
[J].
Liquid-phase sintering of lead halide perovskites and metal-organic framework glasses
[J].[Figure: see text].
Coordination-network-based ionic plastic crystal for anhydrous proton conductivity
[J].An ionic coordination network consisting of protonated imidazole and anionic one-dimensional chains of Zn(2+) phosphate was synthesized. The compound possesses highly mobile ions in the crystal lattice and behaves as an ionic plastic crystal. The dynamic behavior provides a proton conductivity of 2.6 × 10(-4) S cm(-1) at 130 °C without humidity.
Investigating the melting behaviour of polymorphic zeolitic imidazolate frameworks
[J].
Metal-organic framework crystal-glass composites
[J].The majority of research into metal-organic frameworks (MOFs) focuses on their crystalline nature. Recent research has revealed solid-liquid transitions within the family, which we use here to create a class of functional, stable and porous composite materials. Described herein is the design, synthesis, and characterisation of MOF crystal-glass composites, formed by dispersing crystalline MOFs within a MOF-glass matrix. The coordinative bonding and chemical structure of a MIL-53 crystalline phase are preserved within the ZIF-62 glass matrix. Whilst separated phases, the interfacial interactions between the closely contacted microdomains improve the mechanical properties of the composite glass. More significantly, the high temperature open pore phase of MIL-53, which spontaneously transforms to a narrow pore upon cooling in the presence of water, is stabilised at room temperature in the crystal-glass composite. This leads to a significant improvement of CO adsorption capacity.
Zeolite CAN and AFI-type zeolitic imidazolate frameworks with large 12-membered ring pore openings synthesized using bulky amides as structure-directing agents
[J].Using bulky amides as the structure-directing agents (SDAs) is an alternative synthetic strategy for the exploration of crystalline large pore (≥12-membered ring) zeolitic imidazolate frameworks (ZIFs). Specifically, by using the bulky amides, dibutylformamide (DBF) and dipropylformamide (DPF) as solvent and imidazole (Im) as a ligand, two ZIFs mimicking the CAN and AlPO-5 (AFI) zeotypes with 12-membered ring (MR) pore openings were synthesized, and denoted as CAN-[Zn(Im)] and AFI-[Zn(Im)], respectively. These two materials are the first known examples of Zn(Im) polymorphs with 12-MR pores and AFI-[Zn(Im)] has the largest pore apertures reported to date for ZIF materials. The concept that the bulky amides used were not simply acting as the solvent, but were in fact acting as SDAs or templates during the synthesis of the large pore ZIFs, was suggested by the closeness of the geometrical fit between the guest DBF and the can cages (composite building units) of the CAN-[Zn(Im)].
Flux melting of metal-organic frameworks
[J].Recent demonstrations of melting in the metal-organic framework (MOF) family have created interest in the interfacial domain between inorganic glasses and amorphous organic polymers. The chemical and physical behaviour of porous hybrid liquids and glasses is of particular interest, though opportunities are limited by the inaccessible melting temperatures of many MOFs. Here, we show that the processing technique of flux melting, 'borrowed' from the inorganic domain, may be applied in order to melt ZIF-8, a material which does not possess an accessible liquid state in the pure form. Effectively, we employ the high-temperature liquid state of one MOF as a solvent for a secondary, non-melting MOF component. Differential scanning calorimetry, small- and wide-angle X-ray scattering, electron microscopy and X-ray total scattering techniques are used to show the flux melting of the crystalline component within the liquid. Gas adsorption and positron annihilation lifetime spectroscopy measurements show that this results in enhanced, accessible porosity to a range of guest molecules in the resultant flux melted MOF glass.
Meltable mixed-linker zeolitic imidazolate frameworks and their microporous glasses: from melting point engineering to selective hydrocarbon sorption
[J].Porous glasses from metal-organic frameworks (MOFs) represent a new class of functional inorganic-organic materials, which have been proposed for applications ranging from solid electrolytes to radioactive waste storage. So far, just a few zeolitic imidazolate frameworks (ZIFs), a subset of MOFs, have been reported to melt and the structural and compositional requirements for MOF melting and glass formation are poorly understood. Here, we show how the melting point of the prototypical ZIF-4/ZIF-62(M) frameworks (composition M(im)(bim); M = Co, Zn; im = imidazolate; bim = benzimidazolate) can be controlled systematically by adjusting the molar ratio of the two imidazolate-type linkers im and bim. By covering the entire range from = 0 to 0.35, we unveil a delicate transition from ZIF materials showing sequential amorphization/recrystallization to derivatives exhibiting coherent melting and a liquid phase that is stable over a large temperature window. The melting point of this ZIF system is a direct function of and can be lowered from ca. 430 °C to only 370 °C, by far the lowest melting point reported for a three-dimensional porous MOF. On the basis of our results, we postulate compositional requirements for ZIF melting and glass formation, which may guide the search for other meltable ZIFs. Moreover, gas physisorption experiments establish that the ZIF glasses adsorb technologically relevant C and C hydrocarbons. Importantly, the adsorption kinetics are much faster for propylene compared to propane and are also dependent on the im:bim ratio, thus demonstrating the potential of these ZIF glasses for applications in gas separation.
New insights into the breathing phenomenon in ZIF-4
[J].
Porous purple glass-a cobalt imidazolate glass with accessible porosity from a meltable cobalt imidazolate framework
[J].
Control of structure topology and spatial distribution of biomacromolecules in protein@ZIF-8 biocomposites
[J].
Opening ZIF-8: a catalytically active zeolitic imidazolate framework of sodalite topology with unsubstituted linkers
[J].A zeolitic imidazolate framework material of SOD topology possessing primarily unsubstituted imidazolate (im) linkers has been synthesized via solvent-assisted linker exchange (SALE) of ZIF-8. The structure of the new material, SALEM-2, has been confirmed through (1)H NMR and powder and single-crystal X-ray diffraction. SALEM-2 is the first example of a porous Zn(im)(2) ZIF possessing a truly zeolitic topology that can be obtained in bulk quantities. Upon treatment with n-butyllithium, the open analogue exhibits Brønsted base catalysis that cannot be accomplished by the parent material ZIF-8. Additionally, it displays a different size cutoff for uptake and release of molecular guests than does ZIF-8.
Thermal amorphization of zeolitic imidazolate frameworks
[J].
Effects of water vapor and trace gas impurities in flue gas on CO2 capture in zeolitic imidazolate frameworks: The significant role of functional groups
[J].
Nanoporous transparent MOF glasses with accessible internal surface
[J].While glassy materials can be made from virtually every class of liquid (metallic, molecular, covalent, and ionic), to date, formation of glasses in which structural units impart porosity on the nanoscopic level remains undeveloped. In view of the well-established porosity of metal-organic frameworks (MOFs) and the flexibility of their design, we have sought to combine their formation principles with the general versatility of glassy materials. Although the preparation of glassy MOFs can be achieved by amorphization of crystalline frameworks, transparent glassy MOFs exhibiting permanent porosity accessible to gases are yet to be reported. Here, we present a generalizable chemical strategy for making such MOF glasses by assembly from viscous solutions of metal node and organic strut and subsequent evaporation of a plasticizer-modulator solvent. This process yields glasses with 300 m(2)/g internal surface area (obtained from N2 adsorption isotherms) and a 2 nm pore-pore separation. On a volumetric basis, this porosity (0.33 cm(3)/cm(3)) is 3 times that of the early MOFs (0.11 cm(3)/cm(3) for MOF-2) and within range of the most porous MOFs known (0.60 cm(3)/cm(3) for MOF-5). We believe the porosity originates from a 3D covalent network as evidenced by the disappearance of the glass transition signature as the solvent is removed and the highly cross-linked nanostructure builds up. Our work represents an important step forward in translating the versatility and porosity of MOFs to glassy materials.
High-porosity metal-organic framework glasses
[J].
Melt-quenched glasses of metal-organic frameworks
[J].Crystalline solids dominate the field of metal-organic frameworks (MOFs), with access to the liquid and glass states of matter usually prohibited by relatively low temperatures of thermal decomposition. In this work, we give due consideration to framework chemistry and topology to expand the phenomenon of the melting of 3D MOFs, linking crystal chemistry to framework melting temperature and kinetic fragility of the glass-forming liquids. Here we show that melting temperatures can be lowered by altering the chemistry of the crystalline MOF state, which provides a route to facilitate the melting of other MOFs. The glasses formed upon vitrification are chemically and structurally distinct from the three other existing categories of melt-quenched glasses (inorganic nonmetallic, organic, and metallic), and retain the basic metal-ligand connectivity of crystalline MOFs, which connects their mechanical properties to their starting chemical composition. The transfer of functionality from crystal to glass points toward new routes to tunable, functional hybrid glasses.