荧光粉转换白光LED的独特性能,包括低能耗、环境友好、使用寿命长、显色指数高等,与传统的荧光灯和白炽灯相比有显著的优势[1,2]。因此,作为第四代照明的LED逐渐取代了传统的照明光源。InGaN LED芯片在近紫外至蓝光区域能高效激发荧光粉,而传统荧光粉在近紫外区的激发能力不强,效率不高,并且不稳定。基于RGB理论将红、绿、蓝三基色荧光粉与紫外LED芯片(UV-LED)结合制备的白光LED,受到荧光粉热稳定性的影响,且三基色荧光粉中红色荧光粉的热稳定性较差。这表明,限制白光LED发展的关键因素,是具有高热稳定性的红色发光荧光粉[3~5]。因此,亟需研发可在近紫外(300~380 nm)-蓝光(420~450 nm)照射下高效激发的新型红光荧光粉。
稀土元素的4f亚层电子结构、原子磁矩大、自旋轨道耦合强、配位数可变和特殊的晶体结构,使其具有独特的光学、电学和磁学性质。Sm3+离子在近紫外-蓝光区域具有优异的光吸收特性,能激发出比Eu3+更丰富的红光[6,7]。由于离子内的4f-4f跃迁被外层的5s2和5p6轨道屏蔽,Sm3+激活的荧光粉能发出较高的光强和色纯度。Ba5Zn4Gd8O21:Sm3+,Ca3TeO6:Dy3+/Sm3+、CaY0.5Ta0.5O3:Sm3+和La6Ba4Si6O24F2:Sm3+已用作固态照明的橙红色荧光粉[8–10]。同时,Sm3+的原材料的价格较低。因此,掺杂Sm3+的橙红色荧光粉,具有极大的潜力和优势[11]。
影响荧光粉发光性能的因素,除了离子内部的跃迁,还有基质材料的结构。ABO3的化学式为单钙钛矿,其中较小的离子B形成角连接的BO6八面体,而较大的A原子位于这些角共享的八面体之间形成的空间中,形成12配位的AO12。掺杂两个电荷与离子大小相差较大的B离子,可形成双钙钛矿A2BB'O6[12]。双钙钛矿的对称和均匀的晶体结构,使其具有稳定的化学性能。双钙钛矿结构中的活化剂和发光离子有序排列,可增强彼此之间的能量传递[13]。Ca2ScNbO6:Mn4+荧光粉具有较高的热猝灭活化能(0.42 eV),Ba2GdSbO6:Eu3+在483 K的发射强度仍保持79.27%,Ca2GdNbO6:Sm3+最佳浓度样品的色纯度为78.38%,在423 K时发射强度仍可以保持在65.32%[14~16]。这表明,具有较高热稳定性的双钙钛矿,可用于稀土离子掺杂荧光粉的结构[17]。鉴于此,本文用高温固相法制备系列Ca2GdSbO6:Sm3+(CGS:Sm3+)橙红色荧光粉,研究其光致发光性能、热稳定性和荧光寿命。
1 实验方法
1.1 样品的制备
实验用原料:CaCO3(AR)、Gd2O3(99.99%)、Sb2O3(99.99%)、Sm2O3(99.99%)、NaF(AR)。
采用高温固相法制备CGS:Sm3+荧光粉。按照Ca2Gd1 - x SbO6:xSm3+(CGS:xSm3+,x = 0、0.01、0.02、0.03、0.04、0.05、0.06)的化学计量比称取上述实验用原料,并加入样品总量4%的NaF作为助熔剂,将其混合后放入玛瑙研钵中充分研磨20 min,然后放入氧化铝坩埚中在1350℃的高温箱式炉中煅烧6 h,将其自然冷却至室温后再次研磨5 min,得到CGS:Sm3+荧光粉。
1.2 性能表征
用X射线衍射仪(XRD,X'Pert PRO,Panalytical)分析样品的物相,辐射源为Cu靶Kα射线(λ = 0.1541 nm),扫描范围为15°~80°,扫描速度为0.02 (°)/s,工作电压为40 kV,工作电流为40 mA。使用荧光粉激发光谱和热猝灭分析系统(EX-1000,Everfine)分析样品的变温光谱、色坐标、色温以及色纯度;用稳态-瞬态光谱仪(FLS980,Edinburgh Instrument)表征样品的激发光谱、发射光谱以及荧光寿命。使用能谱仪(EDS)测定了CGS:0.03Sm3+样品的元素分布。
2 结果和讨论
2.1 CGS:xSm3+ 荧光粉的物相
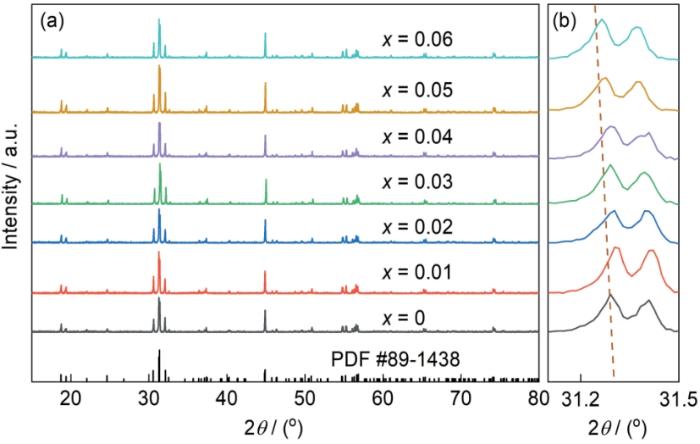
图1a给出了不同掺杂浓度的CGS:xSm3+荧光粉的XRD谱。可以看出,CGS:xSm3+的衍射峰与标准卡片JCPDS No. 89-1438的衍射峰匹配良好,没有杂质的衍射峰,主峰的峰形尖锐。这表明,在此条件下合成的CGS:xSm3+晶体为单相,掺入Sm3+离子没有改变基质的晶体结构。
图1
图1
CGS:xSm3+ (x = 0~0.06)荧光粉的XRD谱和CGS:xSm3+ (x = 0~0.06)荧光粉在2θ = 31.1°~31.5°的放大谱
Fig.1
XRD patterns of CGS:xSm3+ (x = 0.01~0.06) (a)and magnified XRD patterns for the 2θ in the region of 31.1°~31.5° (b)
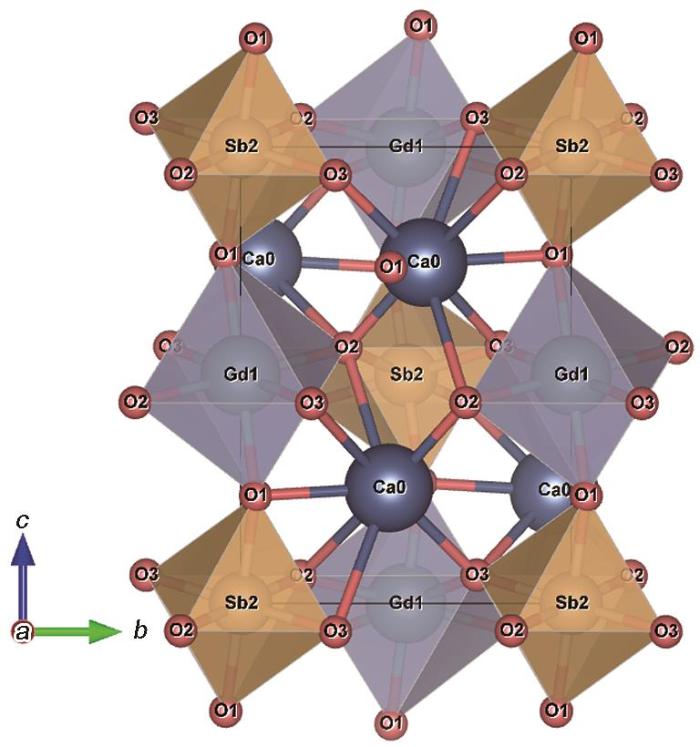
图2表明,CGS属于斜方晶系结构,空间群为P21/n,晶胞参数为a = 0.5597 nm,b = 0.5858 nm,c =0.8095 nm,晶胞体积V = 0.265439 nm³,α = β = 90°,γ = 89.732°。Gd3+和Sb5+分别被六个氧原子包围,形成的GdO6和SbO6八面体在角落共用一个氧原子呈交错排列,丰富的八面体结构为Sm3+离子的掺入提供了最佳位点。
图2
Sm3+离子在CGS基质中的替代状况是:阳离子的半径分别为:Ca2+为0.1 nm,Gd3+为0.094 nm,Sb5+为0.061 nm,Sm3+为0.096 nm,阳离子配位数分别为:Ca2+为6,Gd3+为6,Sb5+为6,Sm3+为6。掺杂阳离子(Sm3+)和宿主阳离子(Ca2+,Gd3+,Sb5+)的尺寸偏差(Dr )为[6]
其中Rm (CN)和Rd (CN)分别为配位数给定时的宿主阳离子和掺杂阳离子的离子半径。由此计算出,Sm3+与Ca2+、Gd3+、Sb5+之间的Dr 值分别为4.0%、2.1%、57.3%。在理论上Dr 值不能超过30%,Sm3+与Ca2+、Gd3+之间的偏差均低于15%。考虑到同价态和配位数优先替代原则,在相同的配位环境下Sm3+能较为容易地替代Gd3+在晶体中的阳离子格位,且不会使CGS的晶体结构发生太大的变化。在图1b中,根据Bragg方程2dsinθ = nλ (d为晶面间距,θ为衍射角,n为衍射级数,λ为波长),对于确定的λ,θ随着d的增大而减小。因此,离子半径较大的Sm3+取代半径较小的Gd3+使晶面间距的增大,使XRD谱向低角度移动。
2.2 CGS:0.03Sm3+ 的表面形貌和元素分布
图3a、b给出了未添加助熔剂的CGS:0.03Sm3+的表面形貌。可以看出,样品颗粒的表面粗糙其尺寸分布在10~20 μm,不利于样品发光的光提取和LED封装。图3c、d给出了添加助熔剂(NaF 4%)样品的表面形貌,可见其表面光滑,尺寸分布在2~12 μm。其原因是,氧化物原料在含F-阴离子的助熔剂中更易熔解。因为NaF的熔点(993℃)较低,氧化物原料未达到反应温度(1350℃)就已经熔化,使其充分混合后进行反应。在NaF助熔剂的作用下聚集的小颗粒通过熔解再结晶相互吞噬并融合,形成表面光滑、更容易研磨成细小颗粒的产物。从图中可见,晶形圆滑度不高的荧光粉较大的反射截面使反射量增大而使出光量降低。在理论上,荧光粉的晶形越接近球形则反射效应越弱,光转换效果越好。
图3
图3
未掺NaF的CGS:0.03Sm3+的SEM图像和掺杂NaF的CGS:0.03Sm3+的SEM形貌
Fig.3
SEM images of undoped NaF CGS phosphor (a, b) andSEM images of NaF-doped CGS phosphor (c, d)
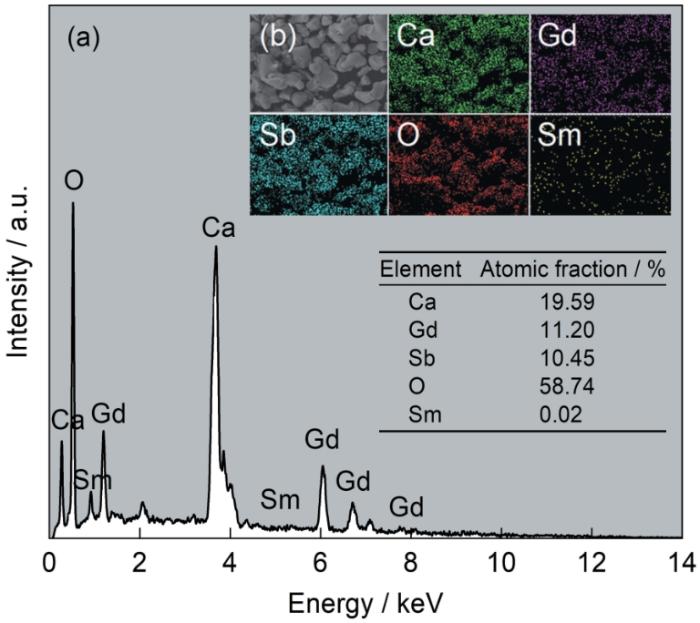
图4a、b分别给出了各个元素的峰值和彩色分布图。可以看出,主要元素Ca、Gd、Sb、O和Sm在荧光粉颗粒中均匀分布,基质的原子百分比接近2∶1∶1∶6,与目标产物相同,EDS能谱中没有杂质峰。这表明,在高温固相反应中Sm3+离子进入Ca2GdSbO6(CGS)的晶格中。
图4
图4
CGS:0.03Sm3+的EDS能谱和CGS:0.03Sm3+荧光粉的元素映射图
Fig.4
EDS spectrum of CGS:0.03Sm3+, (a) and Elemental mapping of the CGS:0.03Sm3+ phosphor (b)
2.3 荧光光谱
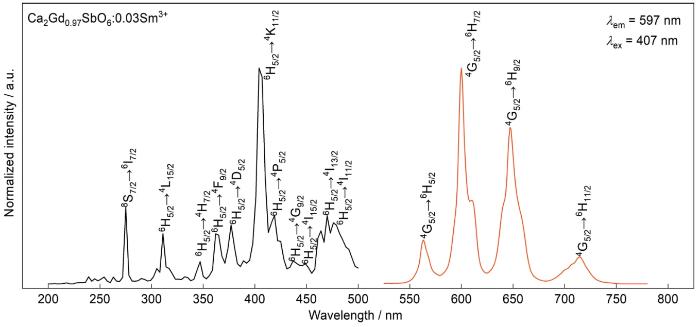
图5给出了CGS:0.03Sm3+荧光粉的激发光谱(PLE,λem = 597 nm)和发射光谱(PL,λex = 407 nm)。
图5
图5
CGS: 0.03Sm3+荧光粉的激发光谱和发射光谱
Fig.5
Excitation spectrum and emission spectrum of CGS: 0.03Sm3+ phosphor
监测波长为597 nm时,样品的激发光谱由一系列200~500 nm的窄带激发峰组成。除了275 nm处的尖峰外,这些激发峰均源自Sm3+离子的电子在4f-4f能级间的跃迁。275 nm的激发峰对应Gd3+的8S7/2→6I7/2,其他位于311 nm、347 nm、362 nm、377 nm、407 nm、419 nm、437 nm、449 nm、470 nm和476 nm处的系列尖峰分别对应Sm3+在6H5/2→4L15/2,6H5/2→4H7/2,6H5/2→4F9/2,6H5/2→4D5/2,6H5/2→4K11/2,6H5/2→4P5/2,6H5/2→4G9/2,6H5/2→4I15/2,6H5/2→4I13/2和6H5/2→4I11/2的吸收跃迁[8,18,19],其中最强的激发在407 nm处,表明CGS:xSm3+能在近紫外光区域激发出较强的橙红光。
激发波长为407 nm时,CGS:0.03Sm3+荧光粉的发射光谱如图中的红色曲线所示,分别由563 nm (4G5/2→6H5/2),597 nm(4G5/2→6H7/2),647 nm(4G5/2→6H9/2)和714 nm(4G5/2→6H11/2)的Sm3+特征发射峰组成[20]。根据电偶极和磁偶极跃迁选择定则,4G5/2→6H7/2处的跃迁满足ΔJ = ±1的选择规则,因此是电偶极跃迁所致,4G5/2→6H5/2跃迁由磁偶极跃迁导致[3,21~25]。对比Sm3+离子的磁偶极跃迁和电偶极跃迁的强度,可见其在基质晶格中的格位对称性。比较563 nm(4G5/2→ 6H5/2)磁偶极跃迁和597 nm(4G5/2→6H7/2)电偶极跃迁,可见597 nm处的跃迁较强,表明在CGS:0.03Sm3+荧光粉中电偶极跃迁占主导,Sm3+在基质中的格位对称性较弱。
综上所述,CGS:xSm3+系列荧光粉的激发带覆盖近紫外至蓝光区域,因此CGS:xSm3+系列荧光粉能被紫外LED 芯片激发而发出橙红色光。
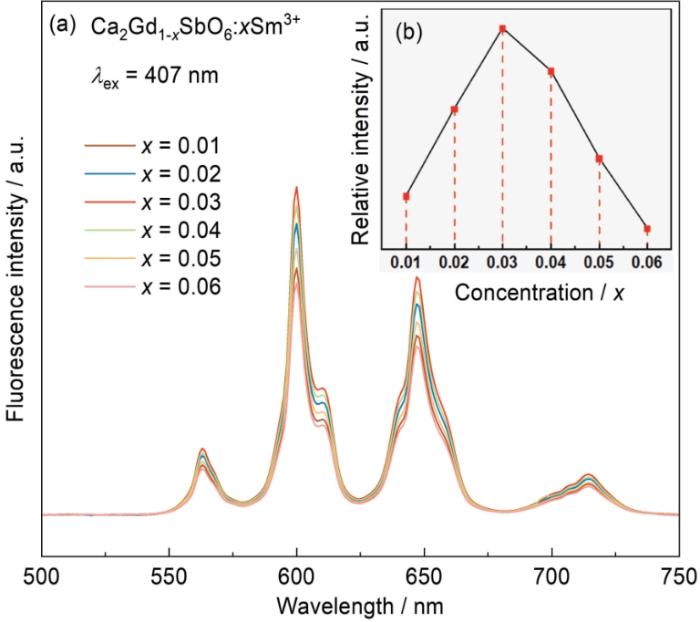
图6a给出了不同掺杂浓度的CGS:xSm3+荧光粉的发射谱,图6b给出了荧光强度与掺杂浓度的关系。从图6a可见,虽然Sm3+掺杂浓度变化,CGS:xSm3+荧光粉发射峰的位置和形状都没有明显的改变。Sm3+掺杂浓度x < 0.03时,荧光强度随着Sm3+掺杂浓度的提高而提高。其原因可能是,激活离子过少的样品中发光中心不足,使发射强度不高;而掺杂浓度x > 0.03时,荧光强度随着Sm3+掺杂浓度的提高而降低。其原因是,掺杂浓度的提高使Sm3+之间的离子间距离变小,引起能量转移使发光强度降低[26],进而使生成的CGS:xSm3+荧光粉发生浓度猝灭。根据Dexter能量转移原理(Dexter excitation transfer)可解释浓度猝灭。电子交换机制产生激发能的转移,即离子间的交叉弛豫(CR)引起浓度猝灭。图7表明,可能存在交叉驰豫通道。荧光粉中可能存在的交叉弛豫通道分别为(4G5/2→6F5/2)→(6H5/2→6F11/2),(4G5/2→6F11/2)→(6H5/2→6F5/2)和4G5/2+6H5/2→26F5/2[27]。随着Sm3+浓度的提高Sm3+离子之间的距离减小,Sm3+占据越来越多的非反演对称中心位置使交叉弛豫逐渐增强,样品的发光强度随之提高。这表明,交换作用和电多极相互作用实现了激活离子间的能量传递,并且能量传递的形式与激活离子之间的距离有关。只有离子间的临界距离Rc < 0.5 nm时离子之间才能进行能量交换。这一临界距离为
图6
图6
CGS:xSm3+ (x = 0.01~0.06)荧光粉的发射谱和主荧光峰发射强度与Sm3+掺杂浓度的关系
Fig.6
PL spectra of CGS:xSm3+ (x = 0.01~0.06) (a) and dependence of integrated fluorescence in tensities of main bands on Sm3+ contents (b)
图7
式中Rc为离子间能量交换的临界距离,xc为掺杂离子的临界浓度值,V为CGS的晶胞体积,Z为CGS的单位晶胞中可以被Sm3+替代的阳离子个数。对于CGS:0.03Sm3+,xc = 0.03,V = 58.824 nm,Z = 2,可计算出临界距离Rc = 2.6554 nm,远远大于0.5 nm。这表明,Sm3+浓度提高引起的猝灭并不是激活剂离子之间的交换作用所致,即引起激活离子发生浓度猝灭的原因只能是电多极能量传递。
根据Dexter理论,荧光发射强度I与稀土掺杂浓度x之间的关系为[28]
式中I为荧光发射强度,x为稀土离子掺杂浓度,K和β为同一激发态下的常数,Q为相互作用类型。Q为6、8和10时分别对应电偶极-电偶极、电偶极-电四极和电四极-电四极相互作用[29]。
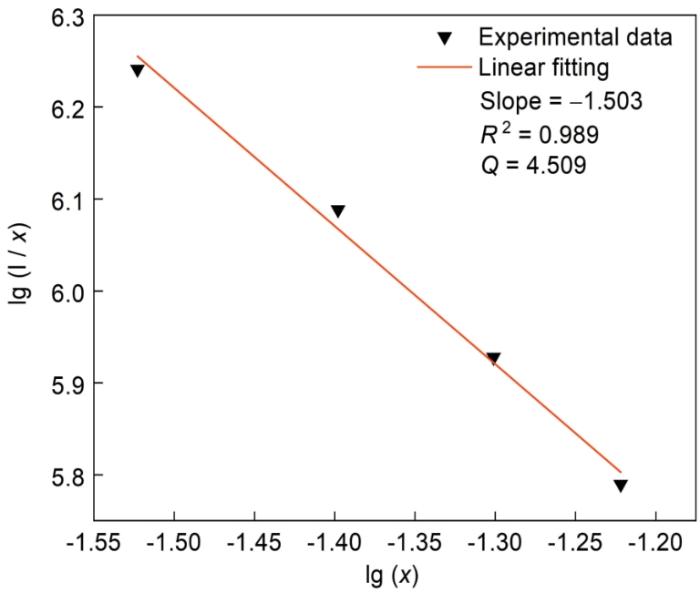
图8给出了CGS:xSm3+橙红色荧光粉的荧光强度lg(I/x)与掺杂离子浓度的对数lg(x)的关系。对曲线拟合可得斜率(Q/3)为-1.503,即Q值为4.509接近于6,表明CGS:xSm3+橙红色荧光粉的离子间相互作用类型为电偶极-电偶极。
图8
图8
CGS:xSm3+(x = 0.01~0.06)中Sm3+的荧光强度lg(I/x)与掺杂离子浓度lg(x)的关系
Fig.8
Correlation between fluorescence intensity lg(I/x) and doping ion concentration lg(x) of CGS:xSm3+(x = 0.01~0.06) phosphors
2.4 荧光衰减
图9
图9
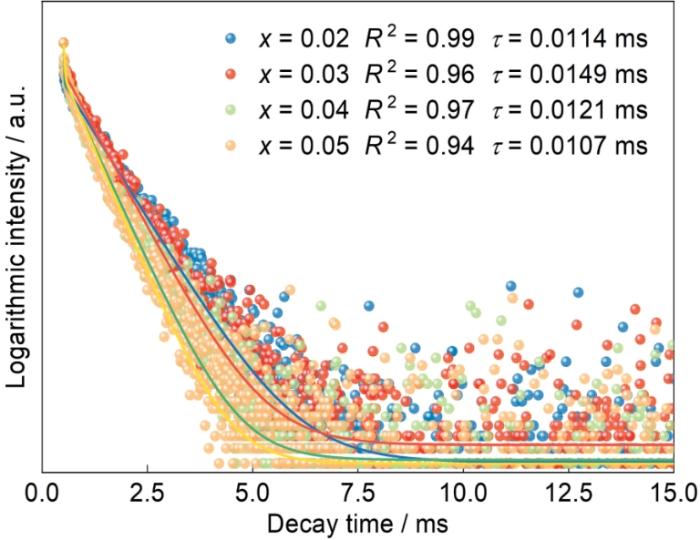
在597 nm处用407 nm激发的荧光粉CGS:xSm3+ (x = 0.02~0.05)的Sm3+衰减曲线
Fig.9
Sm3+ emission of CGS:xSm3+ (x = 0.02~0.05) phosphors excited by 407 nm and monitored at 597 nm
式中I(t)为对应t时的发光强度,I0为t = 0时的发光强度,A1、A2为常数,τ1和τ2为指数分量的衰减时间。平均寿命为
2.5 热猝灭性能
图10a给出了在407 nm光激发下CGS:0.03Sm3+荧光粉在不同温度下的发射光谱。可以看出,四个发射峰的位置没有明显的变化,只是荧光强度随着温度的升高而降低。
图10
图10
CGS:0.03Sm3+荧光粉在不同温度下的荧光发射谱和归一化发射强度
Fig.10
Fluorescence emission spectrum at different temperatures (a) and normalized emission inten-sity (b) of the CGS:0.03Sm3+ phosphor
进一步研究了CGS:0.03Sm3+的热猝灭活化能(∆E)[36]
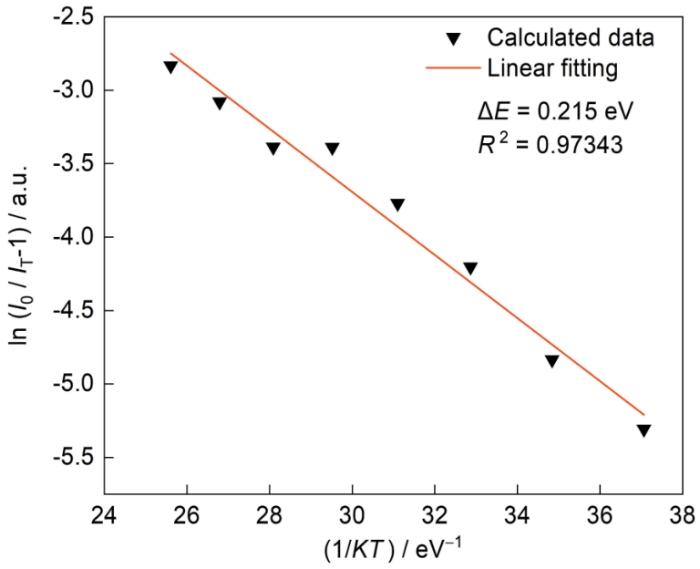
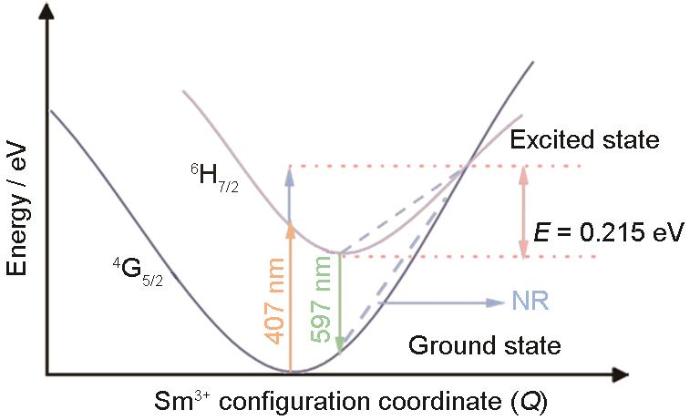
式中I0 为室温下的荧光粉的荧光强度,I 为测量温度下的荧光强度,A为常数,K为波尔兹曼常数(8.62 × 10-5 eV),T为测量时的温度,∆E为活化能。图11给出了橙红色荧光粉CGS:xSm3+的ln(I0/I-1)与1/KT之间的关系,线性拟合后得到斜率为-0.215,即活化能(ΔE)为0.215 eV。与其他研究结果相比,Ca2LaNbO6:Sm3+的活化能ΔE为0.214 eV,Ca2GdNbO6:Sm3+的活化能ΔE为0.2 eV,YPO4:Sm3+的活化能∆E为0.2566 eV。这表明,CGS:xSm3+橙红色荧光粉有良好的热稳定性。
图11
用图12给出的CGS:Sm3+的位形坐标图,能进一步解释CGS:Sm3+的热猝灭。根据荧光粉的热猝灭原理,在较低温度下受到激发的电子从激发态6H7/2回到基态4G5/2能级并发出橙红光(597 nm),而随着温度的升高晶格振动能量增大,一部分电子吸收能量后跃迁到6H7/2与4G5/2的交点,处于激发态的电子吸收能量后发生无辐射跃迁回到基态4G5/2,从而产生了热猝灭。以上结果表明,猝灭温度与激活能∆E成正比。
图12
2.6 色坐标
图13
图13
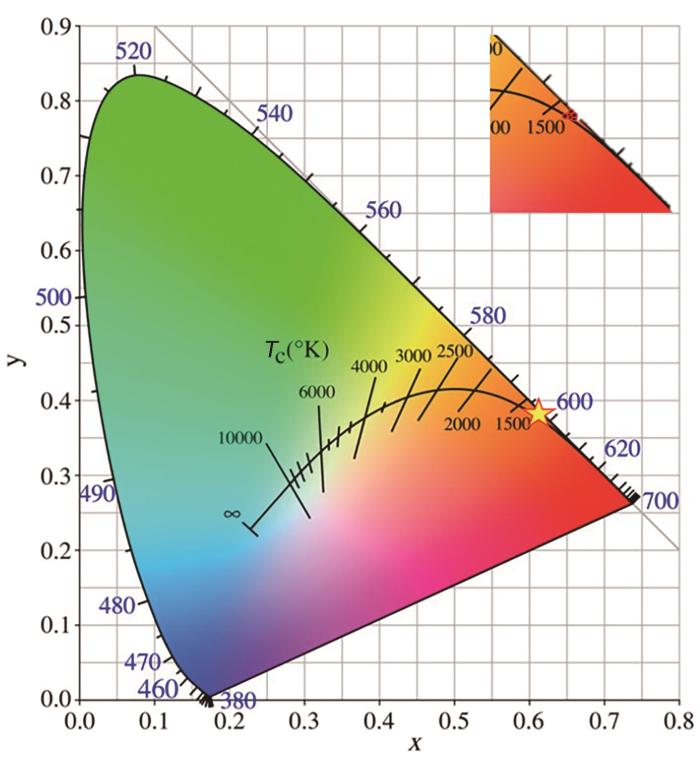
荧光粉CGS:xSm3+ (x = 0.01~0.06)的CIE色坐标
Fig.13
CIE chromaticity coordinates of CGS:xSm3+ (x = 0.01~0.06)
其中(xi,yi )、(xd,yd )和(x,y)分别对应NTSC (National television standards committee)定义的白光光源坐标(0.3101,0.3162)、荧光粉主波长的CIE色度坐标和CGS:xSm3+ (x = 0.01~0.06)的CIE色度坐标。CGS:xSm3+ (x = 0.01~0.06)的相关色温(CCT)可使用McCamy经验公式[37]
计算。其中a = -437, b = 3601, c = -6861, d = 5514.32, n = (x-0.3320)/(y-0.1858)。
表1 荧光粉CGS:xSm3+ (x = 0.01~0.06)的色坐标、相关色温和色纯度
Table 1
| Sm3+ concentration (x) | CIE chromaticity coordinates (X, Y) | Color purity / % | Correlated color temperature / K |
|---|---|---|---|
| 0.01 | (0.6156, 0.3823) | 99.5 | 1305 |
| 0.02 | (0.6167, 0.3826) | 99.9 | 1301 |
| 0.03 | (0.6146, 0.3826) | 99.3 | 1311 |
| 0.04 | (0.6162, 0.3829) | 99.9 | 1305 |
| 0.05 | (0.6151, 0.3824) | 99.4 | 1308 |
| 0.06 | (0.6158, 0.3832) | 99.9 | 1308 |
3 结论
(1) 用高温固相法可合成Ca2Gd1-xSbO6:xSm3+橙红色荧光粉,Sm3+掺杂的最佳浓度为x = 0.03,其浓度猝灭由电偶极-电偶极相互作用主导。
(2) 在407 nm激发波长下,根据Sm3+在597 nm处4G5/2→6H7/2的跃迁发射,样品能发射较强的橙红色,最佳掺杂浓度的色坐标为(0.6146,0.3826),色纯度约为99.3%,荧光寿命都在0.015 ms以下且随着掺杂离子浓度增加荧光寿命变短。
(3) 这种新型橙红色荧光粉Ca2GdSbO6:Sm3+具有较高热稳定性和较短的荧光寿命。
参考文献
Research challenges to ultra-efficient inorganic solid-state lighting
[J].
Luminescence and Energy Transfer of Tunable Emission Phosphor Ca9Al(PO4)7: Tb3+, Sm3+ for White LEDs
[J].
白光LEDs用颜色可调型荧光粉Ca9Al(PO4)7: Tb3+, Sm3+的发光及能量传递
[J].
Synthesis, structure and photoluminescence properties of europium-, terbium-, and thulium-doped Ca3Bi(PO4)3 phosphors
[J].
Preparation and luminescence properties of Eu3+ activated K2CaP2O7 phosphors
[J].
Eu3+激活K2CaP2O7荧光粉的制备和发光性质
[J].
Synthesis red-emitting Ca2LaNbO6:xSm3+ phosphors for good color-rendering-index white-LED devices
[J].
Preparation and Luminescence Properties of Reddish-orange Phosphors Ca3Y2Si3O12: Sm3+
[J].
橙红色荧光粉Ca3Y2Si3O12:Sm3+的制备及发光特性
[J].
Near-ultraviolet excited down-conversion Sm3+-doped Ba5Zn4Gd8O21 reddish-orange emitting nano-diametric rods for white LEDs
[J].
Orange-red-emitting Sm3+-doped double perovskite CaY0.5Ta0.5O3 phosphor with highly thermal stability for white LED applications
[J].
La6Ba4Si6O24F2: Sm3+ novel red-emitting phosphors: Synthesis, photoluminescence and theoretical calculations
[J].
Preparation of BaWO4: Sm3+ red phosphor by microwave method and its luminescent properties
[J].
SrWO4:Sm3+荧光粉的微波合成及发光性能研究
[J].
Double perovskite Cs2AgInCl6: Cr3+: broadband and near-infrared luminescent materials
[J].
A novel Ca2ScNbO6:Eu3+ phosphor with excellent thermal stability for high color rendering WLED
[J].
Novel deep‐red‐emitting double‐perovskite type Ca2ScNbO6:Mn 4+ phosphor: Structure, spectral study, and improvement by NaF flux
[J].
Strong red emission with excellent thermal stability in double-perovskite type Ba2GdSbO6:Eu3+ phosphors for potential field-emission displays
[J].
Synthesis and luminescence properties of reddish-orange-emitting Ca2GdNbO6:Sm3+ phosphors with good thermal stability for high CRI white applications
[J].
Preparation and Characterization of Double Perovskite Sr2GdSbO6: Eu3+ Phosphor
[J].
双钙钛矿结构Sr2GdSbO6:Eu3+荧光粉的制备与表征
[J].
Luminescence properties of phosphate phosphors Ba3Gd1-x(PO4)3:xSm3+
[J].
Near-ultraviolet absorption spectra and crystal-field analysis of Gd3+ in Na3[Gd(C4H4O5)3]⋅2NaClO4⋅6H2O
[J].
Research on new orange-red emitting Sm3+-doped NaSr2Nb5O15 phosphors
[J].
Sm3+掺杂NaSr2Nb5O15的新型橙红色荧光粉
[J].
Transition selection rules of rare-earth in optical materials
[J].
稀土离子激活发光材料中能级跃迁的选择定则
[J].
Synthesis and luminescence properties of a novel red Ca0.5Sr0.5MoO4:Sm3+ phospher
[J].
新型红色荧光粉Ca0.5Sr0.5MoO4:Sm3+的制备及光学性能
[J].
Novel orange-red-emitting Sr3Ga2Ge4O14:Sm3+ phosphors with low thermal quenching
[J].
低热猝灭新型Sr3Ga2Ge4O14: Sm3+橙红色荧光粉
[J].
JOES: An application software for Judd-Ofelt analysis from Eu3+ emission spectra
[J].
Characterizations and optical properties of Sm3+-doped Sr2SiO4 phosphors
[J].
Photoluminescence properties of a new orange-red phosphor Sr4Nb2O9:Sm3+
[J].
新型橙红色荧光粉Sr4Nb2O9:Sm3+的光致发光性能研究
[J].
Absorption and fluorescence properties of Sm3+ ions in fluoride containing phosphate glasses
[J].
A novel deep red-emitting phosphor KMgLaTeO6: Mn4+ with high thermal stability and quantum yield for w-LEDs: structure, site occupancy and photoluminescence properties
[J].
Excellent photoluminescence and cathodoluminescence properties in Eu3+-activated Sr2LaNbO6 materials for multifunctional applications
[J].
A novel orange-red emitting phosphor Sr3Lu(PO4)3:Sm3+ for near UV-pumped white light-emitting diodes
[J].
Photoluminescence studies on Eu2+-activated Li2SrSiO4 a potential orange-yellow phosphor for solid-state lighting
[J].
Synthesis, color-tunable emission, thermal stability, luminescence and energy transfer of Sm3+ and Eu3+ single-doped M3Tb(BO3)3 (M = Sr and Ba) phosphors
[J].
Abnormal thermal quenching behavior and optical properties of a novel apatite-type NaCa3Bi(PO4)3F:Sm3+ orange-red-emitting phosphor for w-LED applications
[J].
On the decay time of upconversion luminescence
[J].In this study, we systematically investigate the decay characteristics of upconversion luminescence (UCL) under anti-Stokes excitation through numerical simulations based on rate-equation models. We find that a UCL decay profile generally involves contributions from the sensitizer's excited-state lifetime, energy transfer and cross-relaxation processes. It should thus be regarded as the overall temporal response of the whole upconversion system to the excitation function rather than the intrinsic lifetime of the luminescence emitting state. Only under certain conditions, such as when the effective lifetime of the sensitizer's excited state is significantly shorter than that of the UCL emitting state and of the absence of cross-relaxation processes involving the emitting energy level, the UCL decay time approaches the intrinsic lifetime of the emitting state. Subsequently, Stokes excitation is generally preferred in order to accurately quantify the intrinsic lifetime of the emitting state. However, possible cross-relaxation between doped ions at high doping levels can complicate the decay characteristics of the luminescence and even make the Stokes-excitation approach fail. A strong cross-relaxation process can also account for the power dependence of the decay characteristics of UCL.
Morphology formation mechanism and fluorescence properties of nano-phosphor YPO4:Sm3+ excited by near-ultraviolet light
[J].
Ca3La6Si6O24:Eu3+ orange-red-emitting phosphor: Synthesis, structure and luminescence properties
[J].
Correlated color temperature as an explicit function of chromaticity coordinates
[J].
Highly efficient cyan-green emission in self-activated Rb3RV2O8(R = Y, Lu) vanadate phosphors for full-spectrum white light-emitting diodes (LEDs)
[J].