理想的冷喷涂锌涂层其腐蚀电位较低,能为钢结构提供阴极保护和良好的自钝化以延长涂层的使用寿命[14]。但是,锌的电化学活性较高,在腐蚀介质中过高的腐蚀速率使涂层的服役寿命较短[15,16]。在高氯的海洋环境中,冷喷涂纯锌涂层的耐久性能有待进一步提高[17]。为此,研究人员采用各种方法提高涂层的性能。Xiong等[18,19]在Zn粉中添加Al粉制备Zn/Al比为2∶3的冷喷涂Zn-Al复合涂层,使基材的抗腐蚀性能提高。李海祥等[20]将气雾化的Zn粉与Al粉按1∶1的比例混合制备出冷喷涂Zn-50Al的复合涂层,其开路电位稳定在-0.99 V,显示出优异的防护性能。Zhang等[21]用石墨烯包覆Al粉,用低压冷喷涂技术制备Zn-G/Al复合涂层具有更低的腐蚀电位和更高的腐蚀电流密度。石墨烯的高导电性增强了涂层中的Zn与Al相的电连接,进一步提高了涂层的阴极保护作用。Zhang等[22]利用Cu的高塑性变形性和高导电性制备的不同Cu质量分数的冷喷涂Zn-Al-Cu复合涂层,其孔隙率随着Cu含量的提高而降低,使其导电性能和耐蚀性能显著提高。对于锌涂层腐蚀速率过高的问题,Huang[23]认为,锌涂层的腐蚀速率过高的主要原因是Zn与Fe基材之间的电位差过高。为了降低二者之间的电势梯度,添加一定量的Ni粉制备了Zn-Ni复合涂层。含有10%Ni(质量分数)的冷喷涂Zn-Ni复合涂层具有更高的腐蚀电位和更低的腐蚀电流密度,使其使用寿命大幅度延长。
已有的研究涉及物相分布更均匀的冷喷涂锌铝合金涂层的研究较少,尤其是在海水浸泡条件下的腐蚀行为和防护机制方面尚无定论。鉴于此,本文用气雾化方法制备Zn粉与Zn15Al合金粉,再用低压冷喷涂技术制备冷喷涂Zn和Zn15Al合金涂层,研究其在海洋环境中的腐蚀行为和防护机制。
1 实验方法
1.1 实验用材料和涂层的制备
图1
图1
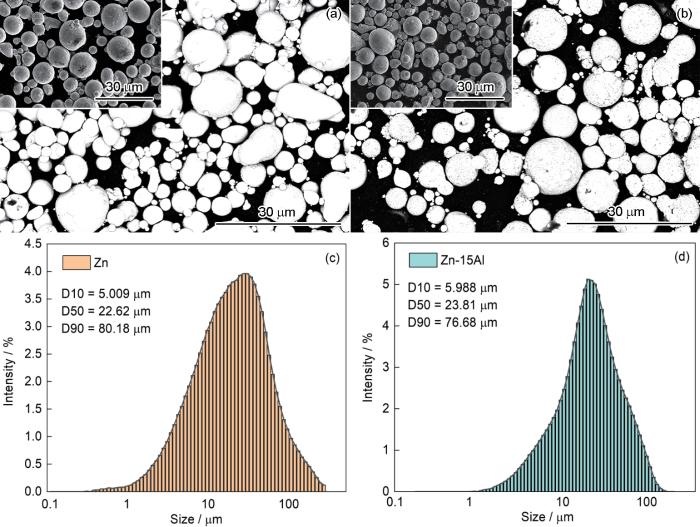
Zn粉和Zn15Al合金粉形貌的二次电子像和背散射像以及Zn和Zn15Al粉体的粒径分布
Fig.1
Morphologies of the Zn (a) and Zn15Al (b) powders observed by SEM backscattering mode (the inset shows the secondary electron image), and the size distribution of Zn (c) and Zn15Al powders (d)
表1 Zn和Zn15Al合金粉的化学成分
Table 1
| Sample | Al | Fe | Si | Pb | Cd | Zn |
|---|---|---|---|---|---|---|
| Zn | 0.0521 | 0.0253 | 0.0395 | 0.0031 | 0.0029 | Bal. |
| Zn15Al | 14.752 | 0.1251 | 0.0895 | 0.0031 | 0.0029 | Bal. |
实验用Q235碳钢基材的化学成分列于表2。使用DYMET 423型冷喷涂设备在Q235碳钢基材表面分别制备冷喷涂Zn和冷喷涂Zn15Al合金涂层。为了使界面结合强度更高,在冷喷涂前用24目的Al2O3白刚玉对Q235基材进行喷砂处理,载气压力为0.7~0.8 MPa,然后用丙酮、乙醇进行超声清洗,干燥后用试样袋封装以避免表面氧化。优化的冷喷涂工艺参数为:压缩空气作为工作气体,载气工作压力设为0.6 MPa,喷涂距离为8 mm,喷枪横向移动速率为300 mm/min,喷涂温度为400℃,送粉速率设为5档。为了便于表述,将制备出的Zn涂层和Zn15Al涂层分别记为CS-Zn和CS-Zn15Al。
表2 Q235碳钢基材的化学成分
Table 2
| Element | C | Mn | Si | S | P | Fe |
|---|---|---|---|---|---|---|
| Content | ≤ 0.12 | ≤ 0.50 | ≤ 0.30 | ≤ 0.04 | ≤ 0.03 | Bal. |
1.2 涂层的形貌观察和成分分析
用Verios 5 UC型场发射扫描电镜(SEM)观察和分析喷涂粉末原材料和涂层腐蚀前后的表面和截面形貌。采用配备的EDS组件半定量分析所选观测区域的元素。为了避免样品制备过程中产生的腐蚀干扰实验结果,用无水乙醇(AR,99.5%,质量分数)为介质制备涂层截面样品。
用D8 Advance X型X-射线衍射仪(XRD)分析喷涂态涂层和腐蚀后涂层腐的蚀产物的物相。扫描角度范围为10°~90°,扫描步长为0.02 (°)/s,扫描速率为4 (°)/min。为了研究涂层腐蚀产物在深度方向上的物相种类与含量差异,使用刀片刮取腐蚀后涂层表面的腐蚀产物。随后,分别测试表层的腐蚀产物和刮取后的块体表面(内层腐蚀产物)的XRD谱。使用Jade 6.5及其自带的JCPDS(ICDD)数据库分析XRD数据。
1.3 电化学测试
电化学测试使用PARSTAT 4000A型电化学工作站进行开路电位、电化学阻抗和极化曲线的测试。
电化学阻抗测试用PMMA管的外径为4 cm,内径为3.57 cm。为了确保样品的一致性,分别用400#,600#,800#,1000#砂纸将涂层表面打磨并减薄至100 ± 10 μm。实验用腐蚀介质采用质量分数为3.5%的NaCl溶液,每2周更换一次。采用三电极系统测试电化学阻抗谱,工作电极为待测涂层,参比电极选用饱和甘汞电极(SCE),辅助电极为ϕ3 cm的铂网电极。测量电化学阻抗谱的扫描频率范围为10-2~105 Hz,交流扰动电压为10 mV。在法拉第电磁屏蔽箱中进行测试,以减少电磁信号的干扰。
测试电化学阻抗谱之前,先测量开路电位,开路电位稳定后测试电化学阻抗谱。使用ZSimpwin软件拟合分析电化学阻抗谱数据。
用F030型平板电解池测试极化曲线,采用三电极体系,参比电极为饱和甘汞电极,辅助电极为20 mm × 20 mm铂网电极,工作电极为正对铂网的1 cm2圆形待测涂层。动电位极化曲线的电压扫描范围为-0.4~0.6 V(vs. Eocp),扫描步幅为0.5 mV,步频为0.5 s。测试极化曲线前先测量开路电位,待开路电位稳定后测试动电位极化曲线。使用Corr-View软件拟合分析测试结果。
2 实验结果
2.1 粉体和涂层的形貌
Zn粉和Zn15Al合金粉的形貌如图1a,b所示,可见气雾化制备的金属粉末大多球形度良好,只有少量的细长颗粒。在背散射模式下可见Zn15Al粉体中浅灰色的Al相均匀分布。粒径分布结果表明,Zn粉的平均粒径D50为22.62 μm,Zn15Al合金粉的平均粒径D50为23.81 μm。
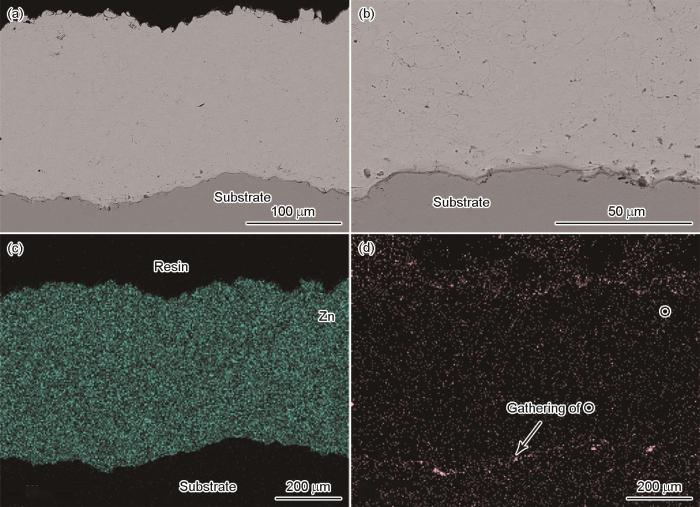
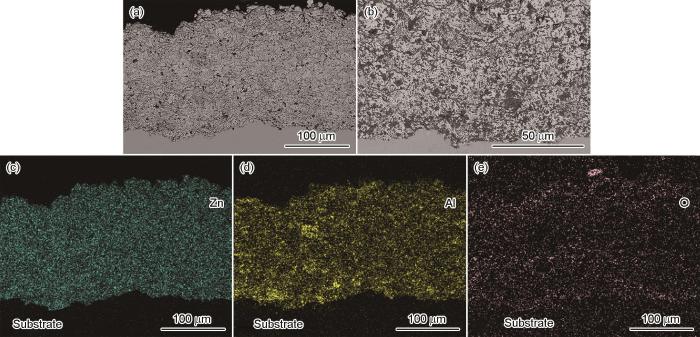
图2和图3给出了CS-Zn和CS-Zn15Al涂层截面的微观形貌和特征元素分布。可以看出,喷砂处理后的基材表面凹凸不平,为涂层提供了机械咬合的支撑点。涂层与基材之间结合紧密,未检测到涂层分层或裂纹等缺陷。涂层的内部致密度高,颗粒之间结合良好,只有少许细小气孔。涂层截面元素分布结果表明,CS-Zn涂层的内部为纯锌,没有发生明显的氧化,在涂层/基材界面处观察到少量O元素富集,可能是喷砂后残留的白刚玉所致;Zn/Al元素在CS-Zn15Al涂层内部均匀分布,只是Al元素出现局部浓度偏高,可能是部分Zn15Al粉末成分偏差所致。此外,在CS-Zn15Al涂层内还观察到少量O元素,可能是粉体中的Al相在空气中生成氧化膜。
图2
图2
CS-Zn涂层截面的形貌和元素分布
Fig.2
Cross-sectional morphologies of the CS-Zn coating (a) coating layer (b) coating/substrate interface, and the corresponding distribution of elements (c) Zn and (d) O
图3
图3
CS-Zn15Al涂层截面的形貌和元素分布
Fig.3
Cross-sectional morphologies of the CS-Zn15Al coating (a) coating layer (b) coating/substrate interface and the corresponding distribution of elements (c) Zn, (d) Al and (e) O
对500倍SEM截面形貌照片,调整图片阈值测定孔隙面积和总面积的比值,使用Image J图像处理软件分析涂层中的孔隙率。为了提高数据的准确性,在每个涂层的不同位置分别截图5个区域。用上述方法计算出CS-Zn和CS-Zn15Al涂层的孔隙率分别为(0.82 ± 0.46)%和(0.69 ± 0.21)%。
图4
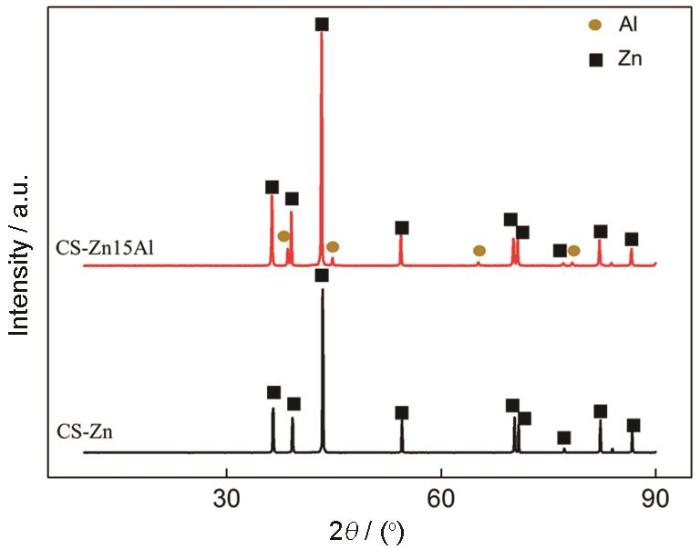
图4
冷喷涂CS-Zn及CS-Zn15Al涂层的XRD谱
Fig.4
XRD pattern of as-deposited CS-Zn and CS-Zn15Al coatings
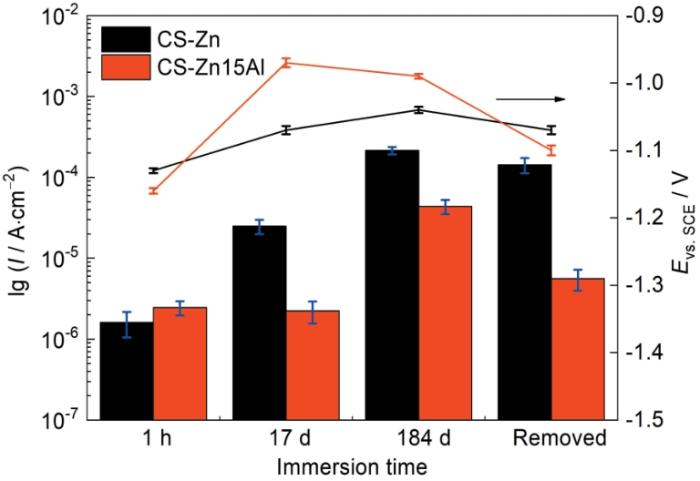
2.2 开路电位和低频阻抗模值
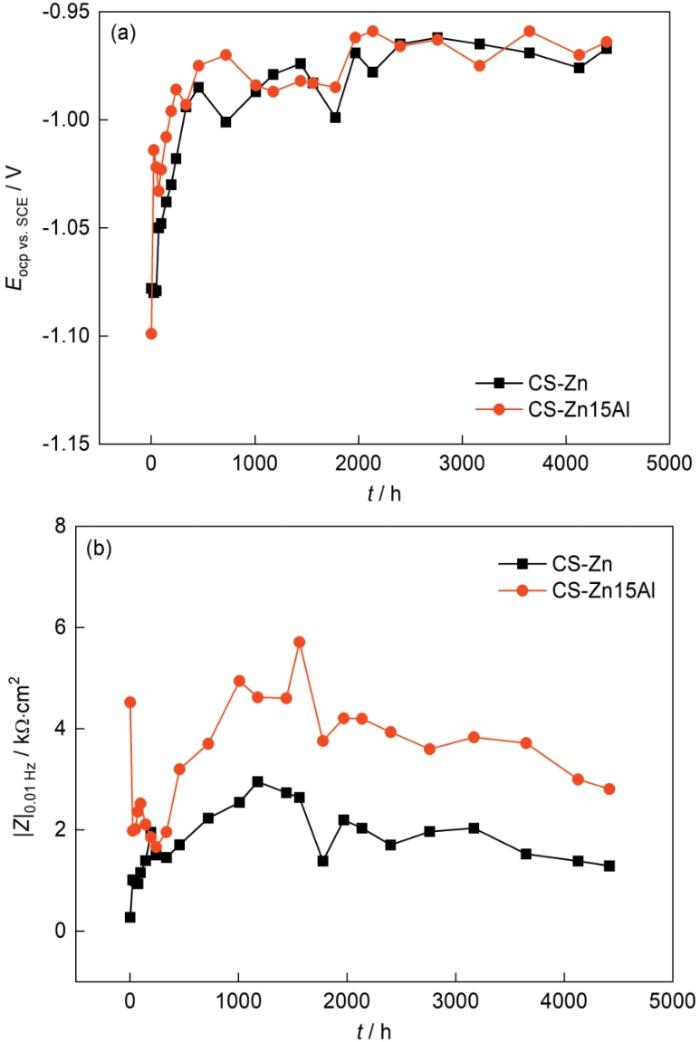
图5a给出了CS-Zn和CS-Zn15Al涂层在3.5%NaCl(质量分数)溶液中的开路电位与浸泡时间的关系。可以看出,两种涂层的开路电位变化趋势基本相同,都是在浸泡初期迅速升高,浸泡1000 h后基本稳定在-0.95 V(vs. SCE)。其原因是,在浸泡早期的腐蚀过程中因Zn具有较高的电化学活性,腐蚀产物迅速生成并堆积在涂层表面形成覆盖层,使腐蚀电位迅速升高。但是,尽管在浸泡后期腐蚀电位趋于稳定,但是仍出现一定幅度的波动。其可能的原因是,涂层表面腐蚀产物的稳定性较差,溶解性较强的腐蚀产物如Zn(OH)2脱落而暴露出新鲜表面。
图5
图5
涂层的开路电位和低频阻抗模值随浸泡时间的变化
Fig.5
Evolution of open circuit potential (a) and low frequency impedance modulus (b) with different immersion time
低频阻抗模值|Z|0.01 Hz是评估涂层耐蚀性能的关键性参数之一[24]。图5b给出了CS-Zn和CS-Zn15Al涂层在3.5%NaCl(质量分数)溶液中浸泡184 d期间低频阻抗模值与浸泡时间的关系。可以看出,CS-Zn涂层的低频阻抗模值随着浸泡时间呈先增大后下降。在浸泡的前1200 h内由2.73 × 102 Ω·cm2逐渐升高至峰值2.95 × 103 Ω·cm2,然后缓慢下降到1.28 × 103 Ω·cm2。与此不同的是,CS-Zn15Al涂层的低频阻抗模值在浸泡初期即呈急速下降的趋势,在浸泡的前240 h期间由初始的2.11 × 103 Ω·cm2下降至最低点2.67 × 103 Ω·cm2,然后随着浸泡时间的延长缓慢增大,在1800 h左右达到峰值5.71 × 103 Ω·cm2,此后逐渐降低,浸泡结束时低频阻抗模值约为2.81 × 103 Ω·cm2。这表明,在整个浸泡周期内CS-Zn15Al涂层的低频阻抗模值|Z|0.01 Hz均显著高于CS-Zn涂层。
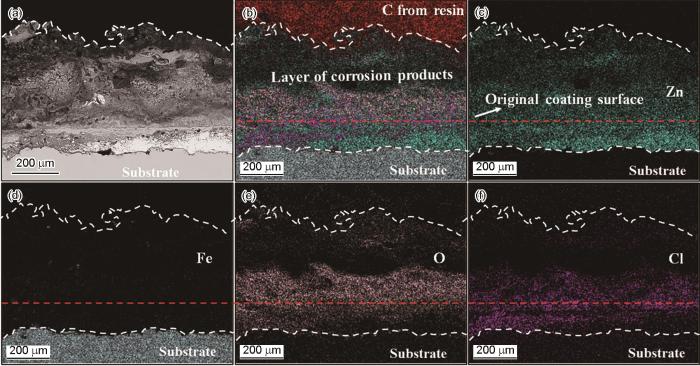
根据上述腐蚀电位和低频阻抗模值的变化,可初步判断CS-Zn15Al涂层比CS-Zn涂层的防护性能更高。进一步从涂层截面形貌观察涂层的腐蚀情况,结果如图6和图7所示。从图6可以看出,CS-Zn涂层在浸泡期间发生了明显的腐蚀,部分基材表面的涂层已完全腐蚀,且涂层表面出现厚度约为300 μm的腐蚀产物层。对涂层截面进行元素面扫分析,结果表明涂层表层的腐蚀产物主要是锌的氧化物,而内层的腐蚀产物则主要有Zn、O和Cl元素。根据锌涂层常见腐蚀产物类型[25],推断涂层表层的腐蚀产物以ZnO为主,而内层的腐蚀产物以Zn5(OH)8Cl2·H2O为主。与CS-Zn涂层相比,CS-Zn15Al涂层保持了较高的完整性,未出现局部完全腐蚀,腐蚀产物主要存在于涂层表面,腐蚀产物层较薄约为50 μm。元素分布结果表明,涂层内部的Zn、Al元素分布与喷涂态相差不大,表层腐蚀产物层主要由O元素与Zn元素组成,并含有少量Al元素及Cl元素。基于此可以推断,涂层表层腐蚀产物主要为Zn的腐蚀产物,Al的腐蚀产物较少。此外,在涂层内部也检测到少量的Cl元素,可能是浸泡后期腐蚀介质通过颗粒间隙或涂层孔隙渗透至基材。根据上述开路电位、低频阻抗模值以及涂层截面形貌与元素分布的结果可以推断,CS-Zn15Al涂层的腐蚀防护性能更高。
图6
图6
浸泡4416 h后CS-Zn涂层界面处的形貌和元素分布
Fig.6
Cross-sectional morphologies of CS-Zn coating and corresponding elements distribution after 4416 h immersion
图7
图7
浸泡4416 h后CS-Zn15Al涂层界面处的形貌和元素分布
Fig.7
Cross-sectional morphologies of CS-Zn15Al coating and corresponding elements distribution after 4416 h immersion
2.3 涂层的腐蚀产物
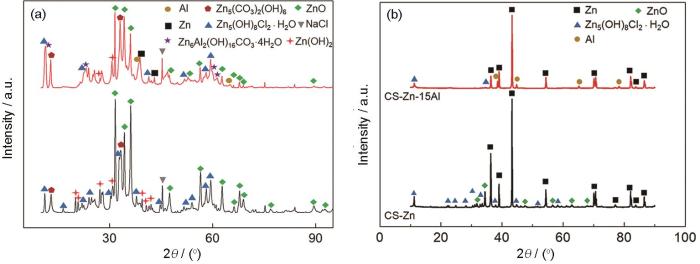
上述截面处的元素分布结果表明,腐蚀产物元素的含量在厚度方向上不同,推测是腐蚀产物的类型和相对含量不同所致。为了进一步对此加以证实,分析两种涂层浸泡4416 h后其表层的腐蚀产物和去除表面产物后内层产物的XRD谱(图8)。从图8a可见,两种涂层的表层腐蚀产物种类基本相同,都有ZnO、Zn5(OH)8Cl2·H2O以及Zn5(CO3)2(OH)6等碱式锌盐。值得注意的是,CS-Zn15Al涂层的表层腐蚀产物中还有Zn6Al2(OH)16CO3·4H2O,但是没有Al2O3。Zn6Al2(OH)16CO3·4H2O是一种典型的层状双氢氧化物(Layered double hydroxides,LDH),其结构通常由两层带正电荷的混合阳离子水滑石结构和中间的水合阴离子构成[26]。由于其特殊的结构以及对阴离子的选择性透过,在吸附、催化、医药以及腐蚀防护领域得到了应用[27]。目前关于Zn-Al LDH的形成机制,仍有较大的争议[28,29],但是普遍认为其对腐蚀因子Cl-有特殊的屏蔽性能[30,31],能大幅提高涂层的防护性能。
图8
图8
浸泡4416 h后涂层腐蚀产物的物相分析
Fig.8
Phase composition characterization of outer (a) and inner (b) layer of corrosion products on of CS-Zn and CS-Zn15Al coating
通常认为,物相的相对含量与XRD谱中衍射峰的强度成正比。由此可以推断,CS-Zn涂层的主要腐蚀产物为ZnO和含量较低的Zn5(OH)8Cl2·H2O和Zn5(CO3)2(OH)6。与其相比,CS-Zn15Al涂层的表层腐蚀产物中的Zn5(OH)8Cl2·H2O和Zn5(CO3)2(OH)6的峰强都显著增强,表明这两种腐蚀产物的含量有所提高。进一步根据对分峰的拟合并结合K值法,可估算出不同腐蚀产物相对ZnO的含量。基于K值法,可得到各物质相对ZnO含量与峰强的对应关系为
其中IZnO和Ii 分别为ZnO和特定产物i的最强峰面积,RZnO和Ri 分别为ZnO与特定产物i的R值。从ICDD库中可查询到,wZnO与wi 分别为ZnO与特定产物i的相对质量分数。
基于上式和分峰拟合结果,CS-Zn涂层腐蚀产物中ZnO的占比最高,Zn(OH)2、Zn5(OH)8Cl2·H2O以及Zn5(CO3)2(OH)6等腐蚀产物相对于ZnO的含量分别为27%,17%以及59%。同样,以ZnO的含量作为参考,CS-Zn15Al涂层表层腐蚀产物拟合计算结果显示Zn(OH)2、Zn5(OH)8Cl2H2O以及Zn5(CO3)2(OH)6相对ZnO的含量分别为13%,117%以及110%。由此可见,CS-Zn15Al涂层中的Al元素大幅度提高了Zn5(OH)8Cl2·H2O和Zn5(CO3)2(OH)6等腐蚀产物的质量分数,同时减少了ZnO的生成。内层产物的XRD物相检测分析结果,如图8b所示。可以看出,内层产物的物相种类和相对含量均与表层显著不同。CS-Zn涂层的内层腐蚀产物有Zn5(OH)8Cl2·H2O和ZnO,用绝热法计算其相对含量分别为wZnO = 46.2%,w
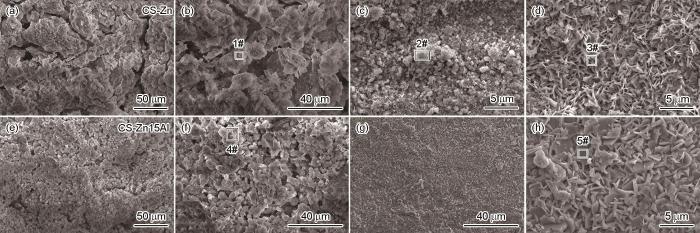
分别观察涂层的表层和内层腐蚀产物的形貌,并分析特征位置的EDS能谱,其结果在图9中给出。图9表明,两种涂层的表层产物(图9a,b,e,f)均为球状堆积成的多孔腐蚀产物层,EDS结果表明两种涂层的表层产物主要含有Zn和O元素。结合上述XRD结果可以推断,该腐蚀产物为ZnO。观察到CS-Zn涂层的内层腐蚀产物的两种形貌:球状(图9c)和片状(图9d)。结合EDS和图8b中XRD谱,可以推断球状腐蚀产物为ZnO,而片状腐蚀产物为Zn5(OH)8Cl2·H2O。CS-Zn15Al涂层内层产物只有一种块状腐蚀产物。根据图8b的XRD谱和表3中的元素含量(4#,5#),可推测这种块状腐蚀产物也是Zn5(OH)8Cl2·H2O。这表明,Al元素的添加不仅影响了涂层腐蚀产物的物相种类和含量,还使Zn5(OH)8Cl2·H2O由片状转变为排列更致密的块状。
图9
图9
浸泡后涂层的表层和内层腐蚀产物的形貌
Fig.9
SEM morphology of outer layer (a, b, e, f) and inner layer (c, d, g, h) of corrosion products
表3 不同位置对应元素EDS结果
Table 3
| Element | 1# | 2# | 3# | 4# | 5# |
|---|---|---|---|---|---|
| Zn | 63.2 | 68.1 | 64.6 | 60.7 | 62.2 |
| O | 36.1 | 31.9 | 25.1 | 33.5 | 21.7 |
| Cl | 0.7 | - | 10.3 | 3.4 | 15.4 |
| Al | - | - | - | 2.4 | 0.7 |
2.4 涂层的极化曲线
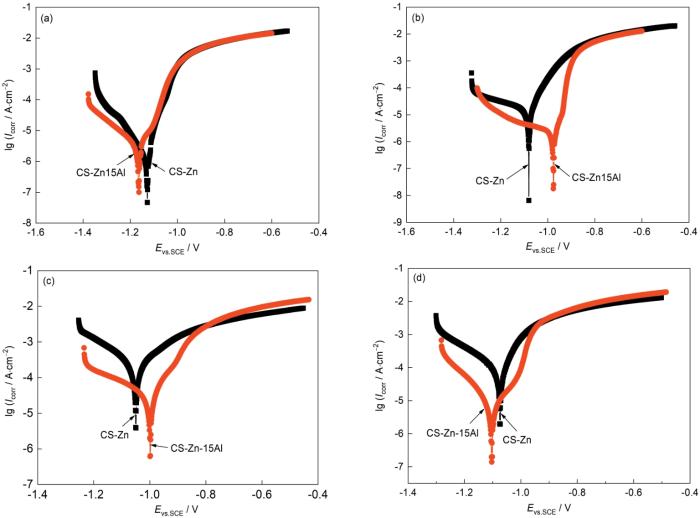
为了进一步验证上述腐蚀产物的类型和含量对涂层防护性能的影响,分别测试浸泡1 h,17,184及184 d去除表层产物后的动电位极化曲线,结果如图10所示。极化曲线拟合结果,如图11所示。从图10a可见,与CS-Zn涂层相比,浸泡1 h 的 CS-Zn15Al涂层具有更高的腐蚀电流密度(Icorr = 2.45 × 10-6 A·cm-2)和更低的腐蚀电位(Ecorr = -1.16 V)。这表明,在浸泡初期CS-Zn15Al涂层的腐蚀倾向和腐蚀速率更高。随着浸泡时间增加到17 d,CS-Zn15Al涂层的Ecorr大幅度升高到-0.97 V,Icorr小幅下降到2.24 × 10-6 A·cm-2;而CS-Zn涂层的Ecorr也小幅升高,由-1.13 V(1 h)升高到-1.07 V(17 d),但是Icorr升高的幅度较大,由1.61 × 10-6 A·cm-2升高到2.49 × 10-5 A·cm-2。随着浸泡时间进一步延长到184 d,CS-Zn涂层的Ecorr进一步升高到-1.04 V,Icorr升高到2.15 × 10-4 A·cm-2;而CS-Zn15Al涂层的Ecorr基本上保持不变,为-0.99 V,Icorr升高到4.38 × 10-5 A·cm-2。上述结果表明,除了在浸泡初期CS-Zn15Al涂层的腐蚀电流密度较高外,在其余浸泡时间CS-Zn15Al涂层都具有更高的腐蚀电位和更低的腐蚀电流密度。可以推测,涂层在浸泡初期生成了保护性腐蚀产物层,提高了CS-Zn15Al涂层的耐蚀性能。这也与上述浸泡试验后对涂层截面的观察结果一致。
图10
图10
浸泡不同时间和表层产物去除后涂层的极化曲线
Fig.10
Potentiodynamic polarization curves of CS-Zn and CS-Zn15Al coatings at different immersion time (a) 1 h, (b) 17 d, (c) 184 d, and (d) after the removal of surface corrosion products
图11
图11
不同浸泡时间极化曲线的腐蚀电流密度与腐蚀电位
Fig.11
The evolution of the fitted results for the measured potentiodynamic polarization curves at different immersion times
为了证实上述腐蚀产物层对涂层的保护作用,测试了去除表层腐蚀产物后涂层的动电位极化曲线,结果如图10d所示。可以看出,将表层产物去除后CS-Zn的腐蚀电流密度小幅度下降到1.43 × 10-4 A·cm-2,腐蚀电位也负移到-1.07 V;而CS-Zn15Al涂层的腐蚀电流密度大幅度下降到5.59 × 10-6 A·cm-2,比去除产物前下降了1个数量级,而腐蚀电位也下降到-1.10 V。两种涂层的表层腐蚀产物层去除后其腐蚀速率都更低。根据上述截面元素分布和XRD谱的结果,ZnO腐蚀产物主要分布在表层产物中,而内层腐蚀产物以Zn5(OH)8Cl2·H2O为主。由此可以推断,ZnO作为腐蚀产物提高了涂层的腐蚀速率。Prosek[32,33]等也认为,ZnO作为半导体材料其导电性较好,降低ZnO的含量有助于降低阴极氧还原反应,提高涂层的耐蚀性能。同时,表层产物去除后CS-Zn15Al涂层的腐蚀电位低于CS-Zn涂层,表明腐蚀产物层覆盖下的涂层仍具有较强的阴极保护作用,更低的腐蚀电流密度意味着其腐蚀速率更低。结合图8b中的XRD谱和图9内层腐蚀产物形貌分析结果,可推测Zn5(OH)8Cl2·H2O作为腐蚀产物降低了涂层的腐蚀速率和提高了涂层的耐蚀性能。片状结构的Zn5(OH)8Cl2·H2O是一种典型的保护性腐蚀产物,能延长腐蚀介质的渗透路径,提高涂层的耐蚀性能[34,35]。
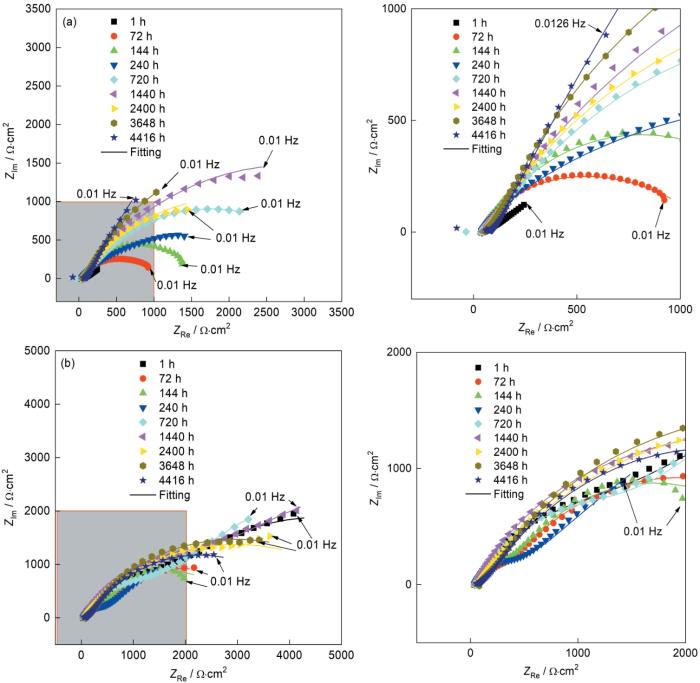
3 涂层的腐蚀行为
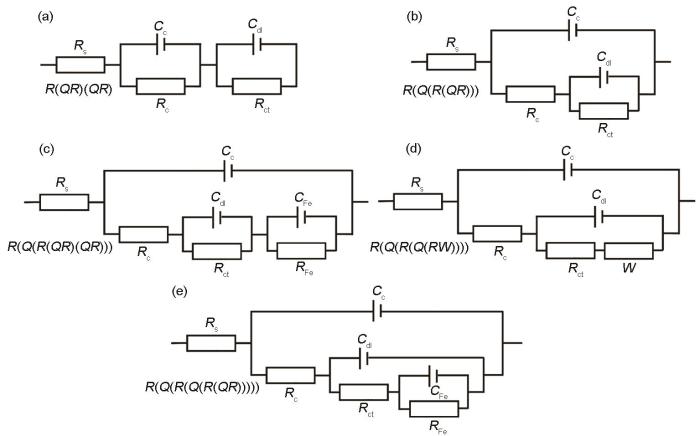
为了进一步分析两种涂层在NaCl溶液中的腐蚀行为,拟合分析不同浸泡时间的EIS结果,结果如图12和图13所示。选用的拟合等效电路,如图14所示,其中Rs为溶液电阻,Rc和Qc分别为涂层阻抗和电容,Rct为电荷转移电阻,Qdl为双电层电容,W为Warburg阻抗,RFe和CFe为与基材腐蚀相关产物的阻抗和电容。图12表明,CS-Zn涂层的容抗弧半径随着浸泡时间的增加呈现先增大后减小的趋势。对阻抗谱拟合分析的结果表明,CS-Zn涂层初始(1 h)等效电路为R(QR)(QR)(图14a),随后迅速转变为R(Q(R(QR)))(图14b)。其原因是,在浸泡初期表层没有腐蚀产物,腐蚀介质均匀到达涂层表面,等效电路为R(QR)(QR);而由于Zn电化学活性高腐蚀较快,在涂层表面迅速生成腐蚀产物,使腐蚀介质的渗透受阻,腐蚀介质与Zn涂层的接触转变为非均匀接触,于是等效电路转变为R(Q(R(QR)))。随着腐蚀的进行多孔腐蚀产物ZnO的含量逐渐提高,腐蚀产物的屏蔽作用被破坏,则浸泡1440 h后等效电路转变为R(QR)(QR)。浸泡时间达2400 h后出现第三个时间常数,对应的等效电路为R(Q(R(QR)(QR)))(图14c),并在随后的浸泡过程中不变。第三个时间常数的出现意味着,腐蚀介质渗透到基材,基材开始参与腐蚀反应。结合涂层截面形貌和电化学阻抗谱可以判断,CS-Zn涂层对腐蚀介质的屏蔽时间为2400 h。
图12
图12
CS-Zn和CS-Zn15Al涂层的Nyquist图随浸泡时间的变化
Fig.12
Evolution of the Nyquist plots of CS-Zn (a) and CS-Zn15Al (b) with different immersion times
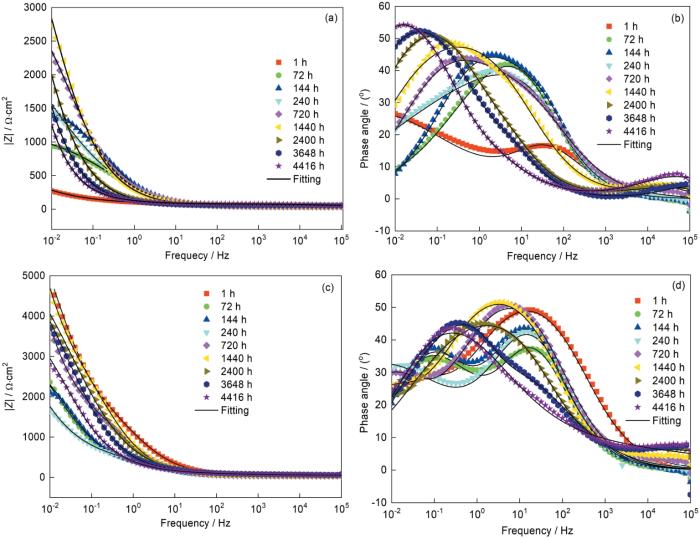
图13
图13
CS-Zn和CS-Zn15Al涂层的Bode图和相位角随浸泡时间的变化
Fig.13
Evolution of Bode plots and phase angle of CS-Zn (a, b) and CS-Zn15Al (c, d) with different immersion times
图14
图14
等效电路示意图
Fig.14
The equivalent circuits used to fit the EIS experimental data obtained at different exposure times (a) initial corrosion stage (b) corrosion stage with corrosion products (c) final corrosion stage for CS-Zn (d) corrosion stage with Warburg impedance (e) final corrosion stage for CS-Zn15Al
在整个浸泡期间CS-Zn15Al涂层的容抗弧半径的变化趋势为:减小-增大-再减小。对阻抗谱的拟合分析发现,在浸泡初期的1~240 h等效电路均为R(QR)(QR),表明腐蚀介质均匀接触CS-Zn15Al涂层表面,因为涂层中的Al相氧化膜提高了涂层耐蚀性,减少了腐蚀产物的生成,对腐蚀介质渗透的屏蔽作用较小。浸泡时间延长到240~2400 h,等效电路转变为R(Q(R(Q(RW))))(图14d),等效电路则由串联转变为并联且出现了Warburg阻抗,表明腐蚀反应由电荷转移控制转变为物质传输控制,腐蚀产物对腐蚀介质的传输有较强的限制,屏蔽作用效果显著。根据腐蚀产物的物相种类和相对含量结果(图8),产物层中的Zn-Al LDH和Zn5(OH)8Cl2·H2O能大幅度提高产物层的屏蔽作用。随着浸泡时间增加到2400~4416 h,等效电路转变为R(Q(R(QR))),因为随着ZnO腐蚀产物的增多腐蚀产物层的致密性被破坏,增加了腐蚀介质的传输通道,使Warburg阻抗消失。浸泡时间增加到4416 h等效电路转变为R(Q(R(Q(R(QR))))),出现了第三个时间常数,表明腐蚀介质渗透至基材和基材开始参与腐蚀反应。
表4 CS-Zn涂层不同时间点的阻抗谱拟合结果
Table 4
| t/ h | Rs/ Ω·cm2 | CPE1 / S·s n ·cm-2 | n1 | Rc/ Ω·cm2 | CPE2 / S·s n ·cm-2 | n2 | Rct/ Ω·cm2 | CPE3 | n3 | RFe/ Ω·cm2 | ChiSq |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 3.4 | 1.37 × 10-2 | 0.42 | 272.3 | 8.34 × 10-4 | 0.69 | 43.1 | - | - | - | 8.16 × 10-4 |
| 72 | 3.5 | 7.54 × 10-4 | 0.41 | 1135 | 3.38 × 10-4 | 0.78 | 27.4 | - | - | - | 3.80 × 10-4 |
| 144 | 3.6 | 6.44 × 10-4 | 0.61 | 1469 | 2.20 × 10-4 | 0.83 | 96.3 | - | - | - | 3.63 × 10-4 |
| 240 | 3.8 | 1.13 × 10-3 | 0.39 | 2012 | 2.92 × 10-4 | 0.76 | 402.2 | - | - | - | 4.17 × 10-4 |
| 720 | 3.5 | 2.64 × 10-4 | 0.81 | 2550 | 9.46 × 10-4 | 0.58 | 1033 | - | - | - | 2.55 × 10-4 |
| 1440 | 4.1 | 1.23 × 10-5 | 0.62 | 3303 | 5.37 × 10-4 | 0.86 | 751.5 | - | - | - | 5.35 × 10-4 |
| 2400 | 4.8 | 2.53 × 10-3 | 0.69 | 2954 | 3.68 × 10-3 | 0.72 | 291.7 | 7.68 × 10-6 | 0.79 | 9.25 | 1.09 × 10-4 |
| 3648 | 4.7 | 2.76 × 10-3 | 0.71 | 2198 | 1.53 × 10-3 | 0.78 | 82.3 | 3.59 × 10-6 | 0.77 | 13.93 | 1.23 × 10-4 |
| 4416 | 4.8 | 6.10 × 10-3 | 0.74 | 1457 | 6.17 × 10-3 | 0.57 | 45.1 | 2.33 × 10-5 | 0.59 | 29.3 | 5.60 × 10-5 |
表5 CS-Zn15Al涂层不同时间点阻抗谱的拟合数据
Table 5
| t/ h | Rs/ Ω·cm2 | CPE1 / S·s n ·cm-2 | n1 | Rc/ Ω·cm2 | CPE2 / S·s n ·cm-2 | n2 | Rct/ Ω·cm2 | W | CPE3 / S·s n ·cm-2 | n3 | RFe/ Ω·cm2 | ChiSq |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 3.4 | 2.12 × 10-4 | 0.68 | 1407 | 9.55 × 10-4 | 0.65 | 6552 | - | - | - | - | 9.32 × 10-4 |
| 72 | 4.6 | 1.97 × 10-4 | 0.77 | 321.5 | 1.48 × 10-3 | 0.62 | 3405 | - | - | - | - | 3.95 × 10-4 |
| 144 | 3.3 | 2.73 × 10-4 | 0.75 | 452.6 | 1.49 × 10-3 | 0.71 | 2614 | - | - | - | - | 5.63 × 10-4 |
| 240 | 3.7 | 2.52 × 10-4 | 0.75 | 439.6 | 2.54 × 10-3 | 0.65 | 1547 | 2.5 × 10-3 | - | - | - | 5.08 × 10-4 |
| 720 | 3.9 | 2.86 × 10-4 | 0.69 | 2170 | 2.09 × 10-3 | 0.69 | 1030 | 1.7 × 10-3 | - | - | - | 6.81 × 10-4 |
| 1440 | 4.1 | 1.32 × 10-4 | 0.58 | 1420 | 1.76 × 10-4 | 0.75 | 3357 | 1.5 × 10-3 | - | - | - | 8.70 × 10-5 |
| 2400 | 4.1 | 2.07 × 10-4 | 0.42 | 361.5 | 2.91 × 10-4 | 0.66 | 6165 | - | - | - | - | 5.19 × 10-4 |
| 3648 | 4.7 | 5.40 × 10-4 | 0.56 | 680.3 | 1.37 × 10-4 | 0.91 | 5290 | - | - | - | - | 5.19 × 10-4 |
| 4416 | 3.9 | 4.98 × 10-4 | 0.59 | 389.2 | 3.11 × 10-4 | 0.82 | 4511 | - | 1.90 × 10-4 | 0.41 | 43.8 | 2.28 × 10-4 |
CS-Zn15Al涂层的Rc先增大后减小,因为涂层的阻抗主要受腐蚀产物的影响。在浸泡初期腐蚀产物较少,涂层的阻抗偏低;随着腐蚀产物的增多尤其是保护性腐蚀产物的生成,阻抗逐渐增大;而随着腐蚀产物ZnO的进一步增加表层腐蚀产物的致密性被破坏,形成开裂/多孔状的产物层,使腐蚀介质更容易渗透,表现为涂层的阻抗降低。在浸泡周期内电荷转移电阻Rct呈先减小后增大再减小的趋势,因为涂层中的Al在空气中生成一层氧化膜[20],在浸泡初期Al相表面的氧化膜作为绝缘层增大了涂层的电荷转移电阻;而随着浸泡时间的增加氧化膜逐渐活化溶解,Rct在浸泡初期呈下降趋势;而随着腐蚀产物的不断增加其钝化作用增加,电荷转移电阻逐渐增大;随着浸泡时间的增加腐蚀产物中ZnO的量增多,其半导体属性降低了电荷转移电阻。与CS-Zn的拟合结果对比,CS-Zn15Al涂层的阻抗Rc更低,因为其腐蚀产物层较薄,约为CS-Zn涂层的1/4;而CS-Zn15Al涂层的电荷转移电阻更高,因为涂层中保护性腐蚀产物的相对质量分数更高,且ZnO的含量更少,产物的钝化作用更显著。
4 结论
(1) CS-Zn涂层的腐蚀是一个加速过程,随着浸泡时间的增加腐蚀速率提高;CS-Zn表层的主要腐蚀产物是ZnO,内层的主要腐蚀产物是Zn5(OH)8Cl2·H2O和ZnO,腐蚀产物ZnO的半导体性质加速涂层的腐蚀反应。
(2) Al元素的添加改变了涂层腐蚀产物的物相种类及相对含量,促进了保护性腐蚀产物Zn5(OH)8Cl2·H2O和Zn-Al LDH的生成并抑制了ZnO的生成;Al的添加引起内层腐蚀产物Zn5(OH)8Cl2·H2O由片状转变为块状,使涂层对腐蚀介质的屏蔽作用显著提高。
(3) Al元素的添加不仅降低了涂层的腐蚀电位使涂层具有更高的耐腐蚀倾向而增强了涂层阴极保护作用,还促进了保护性腐蚀产物的生成,降低涂层腐蚀电流密度,延长了涂层使用寿命,实现涂层防护性能和耐久性能的协同提高。
(4) 可从调控腐蚀产物的角度设计冷喷涂Zn合金涂层,通过成分设计减少ZnO腐蚀产物的生成,调控Zn5(OH)8Cl2·H2O和Zn-Al LDH等保护性腐蚀产物的生长以提高涂层的耐蚀性能。
参考文献
Corrosion protection techniques of marine engineering structure and ship equipment—current status and future trend
[J].
海洋工程结构与船舶的腐蚀防护——现状与趋势
[J].
Marine corrosion and protection: current status and prospect
[J].
海洋腐蚀防护的现状与未来
[J].
Research of preparation and protective performance of Zn-Al alloy coating by low pressure cold spray technology
[D].
低压冷喷涂锌铝合金涂层制备及防护性能研究
[D].
Cold spray coating process for corrosion protection: a review
[J].Cold spray deposition is an emerging technology that addresses many classic shortcomings of more traditional thermal spray processes. Efforts are under way as of this writing to develop a more comprehensive scientific basis for the cold spray process as well as a wider range of suitable materials and its applications for corrosion protection. This paper presents a tutorial introduction to the cold spray coating process, highlighting the coating formation mechanisms, its characteristics and applications. It includes the comparison of cold spray technique with some popular thermal spray techniques. A thorough survey on the combinations of coatings deposited on diverse substrates with their features has also been detailed with emphasis on its applications for oxidation/corrosion protection.
Corrosion behavior of Al(Y)-30%Al2O3 coating fabricated by low pressure cold spray technology
[J].
低压冷喷涂制备Al(Y)-30%Al2O3涂层及其海水腐蚀行为
[J].
The state-of-art of research and development on cold spraying in China
[J].
中国冷喷涂研究进展
[J].
Cold spray technology and its system: research status and prospect
[J].
冷喷涂技术及其系统的研究现状与展望
[J].
A brief review on cold spray coating process
[J].
Formation of interfacial compounds and the effects on stripping behaviors of a cold-sprayed Zn-Al coating on interstitial-free steel
[J].
Properties of Zn-Al-Mg-TiO2 coating prepared by cold spraying
[J].
Contribution in optimization of Zn cold-sprayed coating dedicated to corrosion applications
[J].
State of the research of cold spraying anticorrosion coatings
[J].
冷喷涂制备防腐涂层研究现状
[J].
The use of cold spray coating for corrosion protection
[A].
Improved microstructure and properties of cold sprayed zinc coatings in the as sprayed condition
[J].
Effect of PSS-PEDOT surface-modified Zn particles on anticorrosion performance of acrylic resin coating
[J].Zn particles were surface modified with polystyrene sodium sulfonate doped poly3, 4-ethylene dioxythiophene (PSS-PEDOT). The corrosion performance of the as modified Zn powder was investigated by means of immersion test in 3.5%NaCl (mass fraction) solution and scanning electron microscope, of which the corrosion current density was found one order of magnitude smaller than that of the blank Zn powder. Then, the anticorrosion performance of the coating composed of acrylic resin incorporated with the modified Zn powder was characterized by means of salt spray test and electrochemical impedance spectroscope. It follows that less corrosion of Zn particles on the coating surface was observed and the cathodic protection duration of the coating was prolonged by 20% in contrast with the one incorporated with blank Zn powder. The increased corrosion resistance of Zn particles and enhanced electrical connections that decreased the Zn reactivity was proposed as the possible mechanism of the improved anticorrosion performance of the coating incorporated with modified Zn powder.
PEDOT: PSS改性锌粉对冷涂锌涂层防护性能的影响
[J].
Enhanced corrosion and wear resistance of Zn-Ni/Cu-Al2O3 composite coating prepared by cold spray
[J].
Anticorrosion properties of Zn-Al composite coating prepared by cold spraying
[J].In order to slow down the corrosion and wear of offshore equipment, the Zn–Al composite coating was prepared on Q345 substrate by cold spray technique. The mass fraction of Zn and Al in the raw material was 2:3. The microstructure of the original coating was observed by scanning electron microscopy (SEM) and was characterized by energy dispersive spectrometer (EDS). From the composite alloy coating obtained by cold spraying, it was observed that the Zn and Al particles were uniformly distributed without oxidation product, and the powder particles were significantly plastically deformed. The microstructure of the composite coating is very dense and has strong adhesion to the substrate. Neutral salt spray test (NSS) and electrochemical accelerated corrosion test results showed that Zn–Al composite coating can effectively provide corrosion protection.
Anticorrosion properties of Zn-Al-Mg-ZnO composite coating prepared by cold spraying
[J].Two kinds of composite coatings, Zn[Formula: see text]Al[Formula: see text]Mg5ZnO[Formula: see text] and Zn[Formula: see text]Al[Formula: see text]Mg5ZnO[Formula: see text], were prepared on steel matrix (Q235) by cold spraying. The Zn, Al, Mg, and ZnO in raw materials were calculated by percentage of mass. The morphology of the original coating was observed by scanning electron microscopy (SEM) and characterized by energy dispersive spectroscopy (EDS). It was found that the microstructures of Zn[Formula: see text]Al[Formula: see text]Mg5ZnO[Formula: see text] and Zn[Formula: see text]Al[Formula: see text]Mg5ZnO[Formula: see text] composite coatings prepared by cold spraying were compact without oxidation products, and the plastic deformation of powder particles was significant. The friction and wear test data of the two coatings showed that the Zn[Formula: see text]Al[Formula: see text]Mg5ZnO[Formula: see text] coating had longer-lasting protective properties and wear resistance under the same conditions. Neutral salt spray test (NSS) and electrochemical accelerated corrosion test were carried out on samples at different time periods. The results of samples’ corrosion microstructure and electrochemical curves showed that Zn[Formula: see text]Al[Formula: see text]Mg5ZnO[Formula: see text] and Zn[Formula: see text]Al[Formula: see text]Mg5ZnO[Formula: see text] have good electrochemical protection performance, and Zn[Formula: see text]Al[Formula: see text]Mg5ZnO[Formula: see text] coating has better protection effect.
Microstructure and corrosion behavior of cold-sprayed Zn-Al composite coating
[J].A Zn–Al composite coating was successfully deposited on Q235 steel by cold spray technology for the corrosion protection in the marine atmosphere. The microstructure and corrosion behavior of the prepared coating was studied byScanning Electron Microscope (SEM), X-ray Diffraction (XRD), salt spray test and electrochemical experiments. A 2400-h neutral salt spray corrosion test (with a corrosion medium of 3.5% sodium chloride solution) showed that the prepared cold-sprayed Zn-Al composite coating has excellent anti-corrosion properties. Based on the microstructure evolution and corrosion products analysis, droplets’ flow-driven ‘synergistic corrosion effect’ was proposed to explain the co-corrosion behavior of Zn and Al particles in the composite coating.
Corrosion resistance of cold-sprayed Zn-50Al coatings in seawater
[J].
冷喷涂Zn-50Al复合涂层在海水中的耐蚀性能
[J].利用电化学方法和微观测试手段,研究了冷喷涂 Zn-50Al涂层在天然海水环境中的耐蚀性能。研究表明,与纯锌、纯铝涂层相比,对于钢基体,锌铝复合涂层具有更加合适的阴极保护电位,可以大大提高防护寿命;海水环境中涂层表面会形成一层致密稳定的腐蚀产物膜,能有效阻止腐蚀介质向涂层内部的渗透,涂层会保持在一个较低的腐蚀速度。
Deposition of Zn-G/Al composite coating with excellent cathodic protection on low-carbon steel by low-pressure cold spraying
[J].
Fabrication and characterization of Al-Zn-Cu composite coatings with different cu contents by cold spraying
[J].
Corrosion behavior of low pressure cold sprayed Zn-Ni composite coatings
[J].
Effect of chemical conversion induced by self-corrosion of zinc powders on enhancing corrosion protection performance of zinc-rich coatings
[J].
The effect of surface modification of zinc particles with phosphoric acid on the corrosion resistance of cold galvanizing coatings
[J].
Layered double hydroxide (LDH) for multi-functionalized corrosion protection of metals: a review
[J].Layered double hydroxide (LDH) has been widely developed in the field of corrosion and protection in recent years based on its unique characteristics including anion capacity, anion exchange ability, structure memory effect, and barrier resistance. This paper comprehensively reviews recent work on the preparations, properties of LDH in the forms of powder and film and their applications in different environments in corrosion and protection. Some novel perspectives are also proposed at the end of the review for future research in corrosion and protection field.
Research advance in application of 2D layered double hydroxides (LDHs) in corrosion protection of metals
[J].
二维层状双金属氢氧化物(LDHs)在金属腐蚀防护中应用的研究进展
[J].
Corrosion mechanisms of Zn(Mg, Al) coated steel: the effect of HCO
In situ infrared reflection spectroscopy studies of the initial atmospheric corrosion of Zn-Al-Mg coated steel
[J].
Corrosion resistance and mechanism of one-component organic Zn15Al-rich coating
[J].
Evolution of the surface chemistry of hot dip galvanized Zn-Mg-Al and Zn coatings on steel during short term exposure to sodium chloride containing environments
[J].
Effect of the microstructure of Zn-Al and Zn-Al-Mg model alloys on corrosion stability
[J].
Composition of corrosion products formed on Zn-Mg, Zn-Al and Zn-Al-Mg coatings in model atmospheric conditions
[J].
Effects of al-mg on the microstructure and phase distribution of Zn-Al-Mg coatings
[J].In this work, the composition of the zinc–aluminum–magnesium alloy coating was designed to have a fixed aluminum–magnesium ratio of 1:1, while the content of aluminum and magnesium elements increases gradually within the range of 1–2 wt.%. The micro-morphology of the coating with different compositions was observed by a scanning electron microscope (SEM). Combined with the surface distribution results of energy dispersive spectrometer (EDS) analysis elements and the phase analysis results of diffraction of X-rays (XRD), the phase distribution of the coating is understood. The statistical calculation of the phase distribution was carried out after staining the SEM image by ImageJ, This is consistent with the solidification simulation results of the thermodynamic simulation software (PADAT). The influence of magnesium and aluminum elements on the microscopic morphology and phase distribution of the zinc–aluminum–magnesium (ZnAlMg) coating was studied, and the mechanism of action was analyzed. The results show that the volume ratio of binary eutectic phase (Zn/MgZn2) and ternary eutectic phase (Zn/Al/MgZn2) in the coating tends to increase as the contents of the two elements elevate. The quantity of MgZn2 is the critical factor for the corrosion resistance of the coating; the more MgZn2, the better the corrosion resistance.