非晶合金中的原子是长程无序、短程有序[9~12]。非晶合金兼具催化活性和独特的选择性[13~16]。非晶合金降解废水的优异性能,引起了人们极大的关注。Mg73Zn21.5Ca5.5球磨非晶粉末能在10 min中内将直接蓝6完全降解,其降解效率比球磨铁基非晶粉末高约20倍,比铁基非晶粉末和商用铁粉高1000倍[17]。Fe73Si7B17Nb3非晶合金粉末降解直接蓝6的效率,是商业铁粉的200倍[18]。多数非晶合金降解废水的机制是以Fenton/Fenotn-like为主的高级氧化,依赖活性物质
1 实验方法
1.1 非晶合金丝材的制备
制备非晶合金丝材的原料有高纯钼(99.9%)、铁(99.99%)、钴(99.9%)、硼(99.5%)等单质金属块。用非自耗型真空熔炼炉制备非晶合金母锭,熔炼时用高纯氩气保护。反复熔炼5次以保证合金铸锭的化学成分均匀,合金熔体在水冷铜坩埚内冷却成铸锭。将约1.5 g的锭子装入内径为14 mm的小坩埚中,用WK-Ⅱ型熔融纺丝机制备直径约为60 μm的非晶合金丝材。
1.2 非晶合金丝材的结构和性能表征
将非晶合金丝材样品放入无水乙醇中超声清洗3 min,用玛瑙研钵将其研磨成粒度均匀细小的非晶合金粉末,用XRD-6100型X射线衍射仪测试其XRD谱。测试条件:Cu靶Kα射线照射,衍射角范围30°~80°、扫描速率4 (°)/min。用STA-409PC型差热分析仪(DTA)测试非晶合金样品的玻璃转变温度Tg和晶化开始温度Tx,升温速率为20 K/min,用高纯氩气保护。用Pt电极、饱和甘汞电极和非晶合金丝材组成的标准三电极系统测试非晶合金样品在结晶紫溶液中的动电位极化曲线(扫描速率为0.33 mV/s)和电化学阻抗谱。用钨灯丝扫描电子显微镜(JSM-6610)分析降解染料后丝材样品的表面形貌,并用扫描电子显微镜配备的X射线能谱仪(EDS)分析降解前后样品的微区成分。用AXIS SUPRA+型X射线光电子能谱仪(XPS)测定样品表面元素的价态,所用X射线源为单色Al Kα,所有结果用284.8 eV的标准C峰校准。
1.3 测定非晶合金丝材对染料废水降解性能
用Mo51Co17Fe17B15和Mo51Fe34B15非晶合金丝材降解结晶紫模拟染料废水,以评价其降解性能。将100 mg染料溶解在1 L去离子水中,制备出100 × 10-6原始染料溶液并稀释成浓度分别为(20、30、40、50) × 10-6的四种溶液。用水浴控制溶液的温度,温度稳定后用1 mol/L盐酸和1 mol/L氢氧化钠溶液调节溶液的pH值。将称量好的非晶合金丝(2 g/L)放入染料溶液中并进行磁力搅拌,然后添加H2O2开始降解并计时。在反应过程中,在预定的时间点(如3、5、10、13、15 min等)用注射器抽取3 mL溶液,用紫外可见光光度计(Spectrophotometer U-3310)测量降解后溶液的吸收率,根据其结果绘制非晶合金的降解性能曲线,并根据
计算出反应速率常数kobs以定量评价降解性能。其中C0为染料溶液原始浓度,Ct为t时刻溶液的浓度,kobs为反应速率常数,t为反应时间。采样速度300 nm/min,波长范围为450~700 nm。
2 实验结果和讨论
2.1 非晶合金丝材的结构
图1
图1
Mo51Co17Fe17B15和Mo51Fe34B15非晶合金丝材的XRD谱和DSC曲线
Fig.1
XRD pattern (a) and DSC curve (b) of Mo51Co17-Fe17B15 and Mo51Fe34B15 amorphous alloy wires
2.2 非晶合金丝材对偶氮染料的降解性能
2.2.1 MoCoFeB与MoFeB非晶合金丝材降解性能的比较
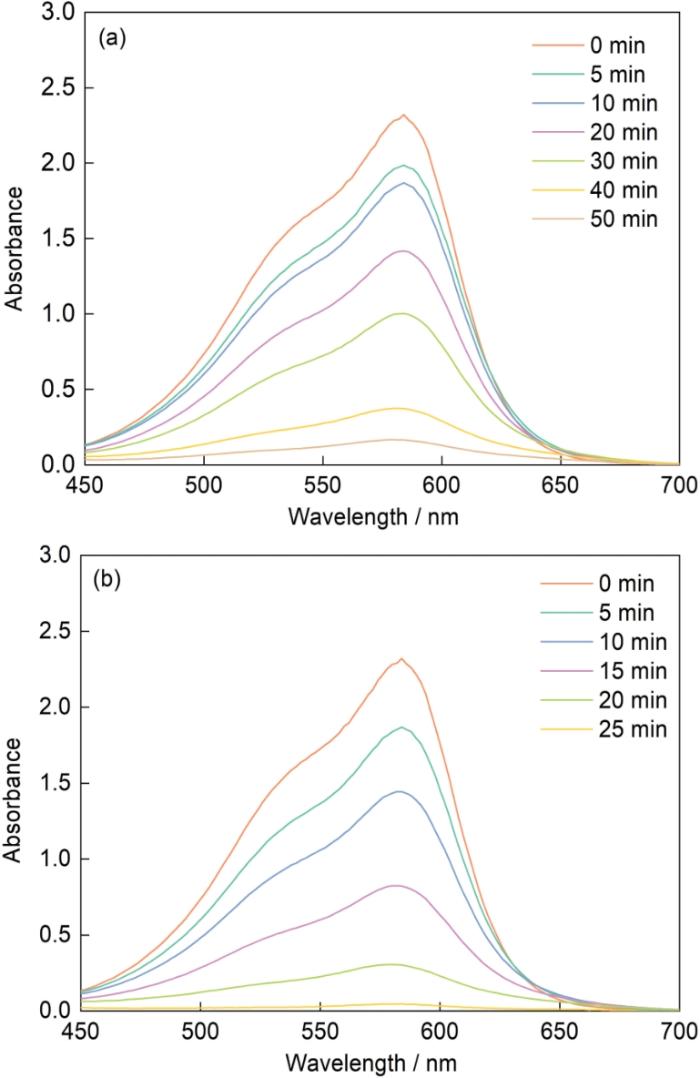
图2给出了Mo51Co17Fe17B15和Mo51Fe34B15非晶合金丝材降解结晶紫溶液后的紫外-可见吸收光谱。可以看出,在波长582 nm处出现了结晶紫染料溶液的最大特征峰。随着反应时间的增加溶液的吸收强度逐渐降低,表明染料中的-N=N-逐渐断裂为-NH2。Mo51Co17Fe17B15非晶丝材在50 min内降解结晶紫溶液的效率为93%,反应速率常数为0.04 min-1 (图2a)。Mo51Fe34B15非晶丝材在25 min内对结晶紫溶液的降解效率为98%,反应速率常数为0.10 min-1(图2b)。由此可见,Mo51Fe34B15非晶合金丝材能在更短的时间内更彻底地降解结晶紫。这表明,Mo51Fe34B15非晶合金丝材对偶氮染料的降解性能优于Mo51Co17Fe17B15非晶合金丝材。
图2
图2
Mo51Co17Fe17B15和Mo51Fe34B15非晶合金丝材降解结晶紫溶液的紫外-可见吸收光谱
Fig.2
UV-Vis absorption profiles of Mo51Co17Fe17B15 (a) and Mo51Fe34B15 (b) amorphous alloy wires degradation Crystal Violet solutions (Room temperature, natural pH, 20 × 10-6 crystal violet solution, 0.1 mol/L H2O2)
2.2.2 染料溶液的pH值对MoCoFeB和MoFeB非晶合金丝材降解偶氮染料性能的影响
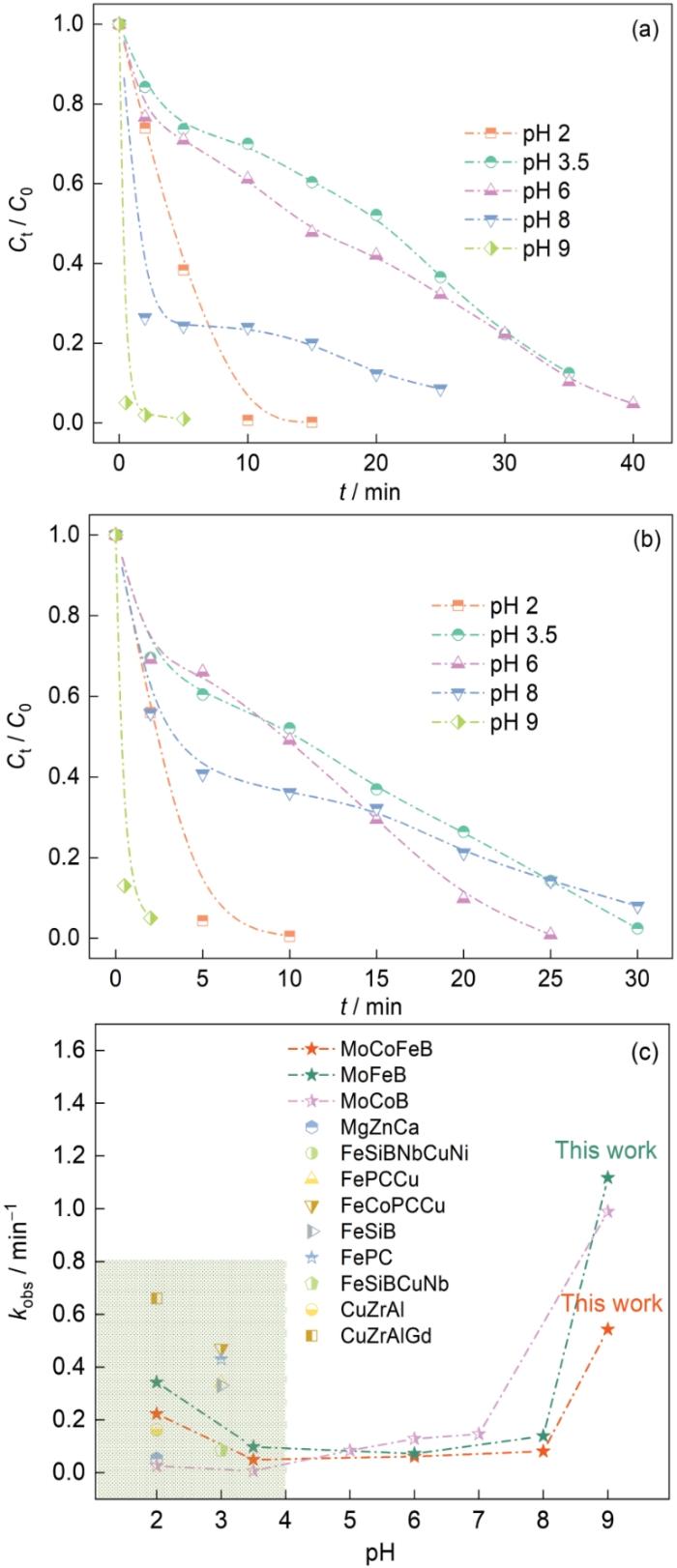
染料溶液pH值的微小变化,对催化剂的降解效率有很大的影响。在pH值为2~9的结晶紫染料溶液中进行Mo51Co17Fe17B15和Mo51Fe34B15非晶合金丝材的降解实验,以研究pH值其降解偶氮染料性能的影响。图3a,b分别给出了Mo51Co17Fe17B15和Mo51Fe34B15非晶合金丝材在不同pH值的溶液中降解的标准化浓度曲线。可以看出,当pH值为2和3.5时,两种非晶合金丝材对结晶紫溶液的降解率均高于99%。这表明,Mo51Co17Fe17B15和Mo51Fe34B15非晶合金具有宽泛的pH值适应性。Fe元素的引入拓宽了Mo基非晶合金在极端酸性介质下的反应活性,使这两种非晶合金丝材在pH值为2~9的宽泛酸碱度下都能使偶氮染料彻底降解。当溶液的pH值从酸性、中性再到碱性的变化过程中,两种非晶合金丝材降解的标准化浓度曲线显示出相同的变化趋势。非晶合金催化剂降解偶氮染料速率先降低后提高,是不同活性物质的主导作用所致。在Mo基非晶合金催化剂降解偶氮染料时[20],
图3
图3
Mo51Co17Fe17B15和Mo51Fe34B15非晶合金丝材降解不同pH值的结晶紫溶液的归一化浓度变化(室温,20 × 10-6结晶紫溶液,0.1 mol/L H2O2)以及不同非晶合金催化剂用于降解偶氮染料废水适用的pH值和反应速率常数kobs[24~30]
Fig.3
Mo51Co17Fe17B15 (a) and Mo51Fe34B15 amorphous (b) alloy wires in different pH values to degrade the normalized concentration of crystal violet solution (Room temperature, 20 × 10-6 crystal violet solution, 0.1 mol/L H2O2) and applicable pH and reaction rate constant kobs (c) of different amorphous alloy catalysts for azo dye wastewater degradation[24~30]
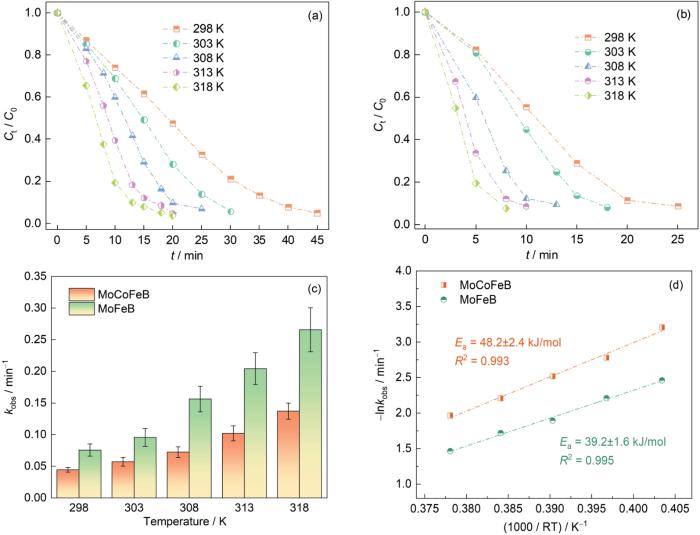
2.2.3 溶液温度对MoCoFeB和MoFeB非晶合金丝材降解偶氮染料性能的影响
计算活化能Ea,其中kobs为反应速率常数,R为气体常数,T为温度,lnA为比例常数。计算结果在图4d中给出。可以看出,Mo51Co17Fe17B15和Mo51Fe34B15在结晶紫溶液中的反应活化能Ea分别为(48.2±2.4) kJ/mol和(39.2±1.6) kJ/mol,均比晶态催化剂(60~250 kJ/mol)的活化能低。其中Mo51Fe34B15非晶丝降解结晶紫溶液的活化能比Mo51Co17Fe17B15非晶丝的低,也比Mo51Co34B15非晶丝的(44.6 kJ/mol)低。这表明,Mo51Fe34B15非晶丝降解染料废水的效率更高。
图4
图4
Mo51Co17Fe17B15和Mo51Fe34B15非晶合金丝材在不同温度下降解结晶紫溶液的归一化浓度变化;反应速率常数kobs以及反应活化能Ea (pH = 2,20 × 10-6结晶紫溶液,0.1 mol/L H2O2)
Fig.4
Mo51Co17Fe17B15 (a) and Mo51Fe34B15 amorphous (b) alloy wires at different temperatures to degrade the normalized concentration of crystal violet solution; reaction rate constant kobs (c) and reaction activation energy Ea (d) (pH = 2, 20 × 10-6 crystal violet solution, 0.1 mol/L H2O2)
2.2.4 H2O2添加量对MoCoFeB和MoFeB非晶合金丝材降解偶氮染料性能的影响
图5给出了H2O2添加量对Mo51Co17Fe17B15和Mo51Fe34B15非晶合金丝材降解性能的影响。与单独使用非晶合金催化剂相比,加入少量的H2O2即可显著提高染料降解效率。其原因是,非晶合金催化H2O2使其分解产生羟基自由基(
图5
图5
Mo51Co17Fe17B15和 Mo51Fe34B15非晶合金丝材不同H2O2添加量降解结晶紫溶液的归一化浓度变化和反应速率常数kobs (303 K,pH = 2,20 × 10-6结晶紫溶液)
Fig.5
Mo51Co17Fe17B15 (a) and Mo51Fe34B15 (b) amorphous alloy wires of different H2O2 addition to degrade the normalized co-ncentration of crystal violet solution and Reaction rate constant kobs (c) (303 K, pH = 2, 20 × 10-6 crystal violet solution)
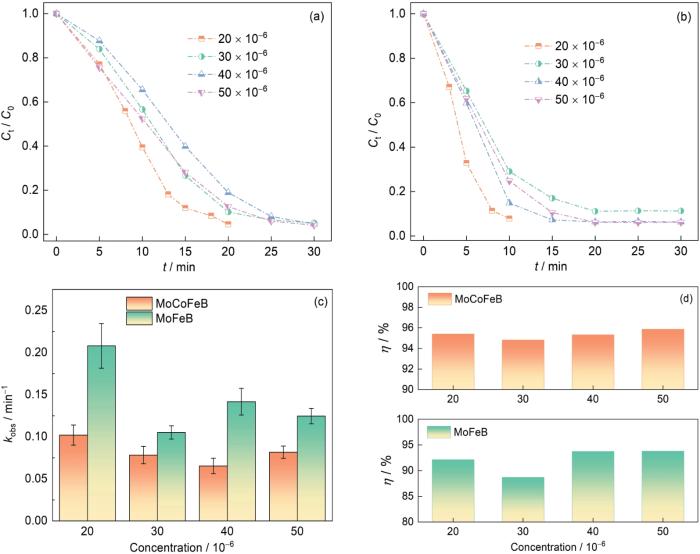
2.2.5 染料溶液的初始浓度对MoCoFeB和MoFeB非晶合金丝材降解偶氮染料性能的影响
图6给出了结晶紫溶液的浓度对Mo51Co17Fe17B15和Mo51Fe34B15非晶合金丝材降解性能的影响。可以看出,随着结晶紫的初始浓度由20 × 10-6提高到50 × 10-6,Mo51Co17Fe17B15对结晶紫的完全降解时间从20 min延长到30 min (图6a),Mo51Fe34B15对结晶紫的完全降解的时间由10 min延长到20 min (图6b),Mo51Co17Fe17B15和Mo51Fe34B15非晶合金丝材的降解速率也不同程度的降低。其原因是,染料的初始浓度较低时大部分染料分子都能接触到活性位点,随着浓度的提高过量的染料分子不能在有限的时间内与活性位点充分反应。但是,在一定的范围内,初始染料浓度的提高使反应速率提高,因为较多的染料分子与非晶合金表面的活性位点碰撞而提高了反应机会。这也是Mo-Fe基非晶丝在高染料浓度(50 × 10-6)结晶紫溶液中降解速率高于低染料浓度(30 × 10-6)的原因。但是,随着浓度的提高反应速率不再随之线性提高,因为活性位点达到了饱和状态(图6c)。初始浓度高达50 × 10-6时Mo51Co17Fe17B15和Mo51Fe34B15丝材都能在30 min内将偶氮键完全断裂,降解效率高达95%以上(图6d)。这意味着,Mo-Fe基非晶合金在宽泛初始浓度范围内依然具有优异的降解性能。
图6
图6
Mo51Co17Fe17B15和 Mo51Fe34B15非晶合金丝材降解不同初始浓度结晶紫溶液的归一化浓度变化以及反应速率常数kobs和降解效率(η = (1-Ct/C0) × 100%) (313 K,pH = 2,0.1 mol/L H2O2)
Fig.6
Mo51Co17Fe17B15 (a) and Mo51Fe34B15 (b) amorphous alloy wires to degrade the normalized concentration of crystal violet solution with different initial concentrations and reaction rate constant kobs (c) and degradation efficiency (η = (1-Ct/C0) ×100%) (d) (313 K, pH = 2, 0.1 mol/L H2O2)
2.2.6 MoCoFeB和MoFeB非晶合金丝材的循环稳定性
非晶合金降解污水,依靠其表面活性位点与污染物的化学反应。但是,非晶合金在长期的反应过程中可能发生腐蚀和钝化。覆盖在合金表面的腐蚀产物减少了活性位点的数量,从而使降解效率降低。这种表面钝化,是限制非晶合金长期稳定性的一个重要因素。Fe基非晶合金降解染料废水时表面附着大量的反应产物,进行酸洗和超声清洗后才能继续使用[7]。同时,随着重复使用次数的增加,降解效率不断降低。图7给出了Mo51Co17Fe17B15和Mo51Fe34B15非晶合金丝材不同循环次数的降解效率。Mo51Co17Fe17B15和Mo51Fe34B15非晶合金丝材的降解效率分别在循环第7、第6次有所降低,第9次循环后其降解效率依然高于86%。与(Fe0.99Mo0.01)78Si9B13非晶相比,Mo-Fe基非晶丝降解染料废水的长期反应活性和重复使用性较为优异[31]。
图7
图7
Mo51Co17Fe17B15和Mo51Fe34B15非晶合金丝材的循环稳定性
Fig.7
Cyclic stability of (a) Mo51Co17Fe17B15 and Mo51-Fe34B15 (b) amorphous alloy wires (at room temperature, pH = 2, 20 × 10-6 crystal violet solution, 0.1 mol/L H2O2)
2.3 非晶合金丝材降解偶氮染料的机理
2.3.1 MoCoFeB和MoFeB非晶合金在偶氮染料溶液中的电化学特性
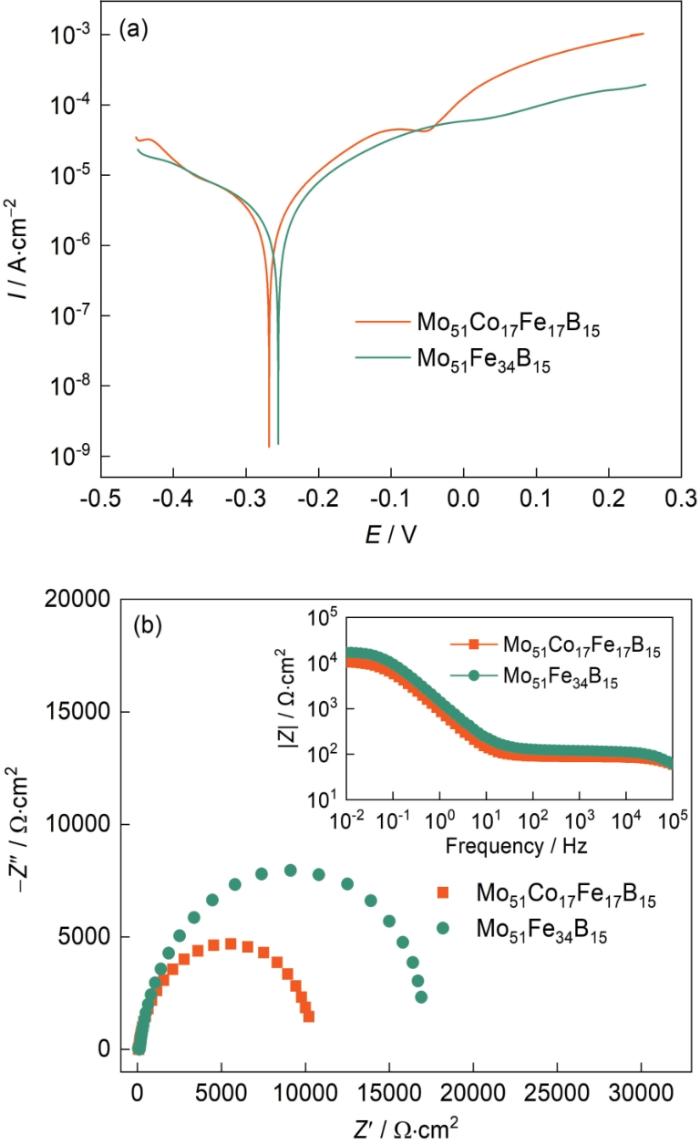
非晶合金的腐蚀性能对其催化降解性能有着重要的影响。非晶合金适当的腐蚀能提高其催化性能,只要不被腐蚀产物覆盖。图8a给出了Mo51Co17Fe17B15和Mo51Fe34B15非晶合金丝材的动电位极化曲线。可以看出,Mo51Co17Fe17B15非晶合金丝材的腐蚀电位为-0.27 V,而Mo51Fe34B15非晶合金丝材的腐蚀电位为-0.25 V。同时,Mo51Co17Fe17B15非晶合金丝材的腐蚀电流密度为3.4 × 10-6 A/cm2,而Mo51Fe34B15非晶丝材的腐蚀电流密度为1.9 × 10-6 A/cm2。具有较低腐蚀电位和较高腐蚀电流密度的Mo51Co17Fe17B15非晶合金丝材比Mo51Fe34B15非晶合金丝材更容易腐蚀。图8b给出了Mo51Co17Fe17B15和Mo51Fe34B15非晶合金丝材在结晶紫染料溶液中的电化学阻抗图。可以看出,这两种非晶合金丝材的奈奎斯特图在高频区都是半圆。与Mo51Co17Fe17B15非晶合金丝材相比,Mo51Fe34B15非晶合金丝材的半圆半径较大,表明其具有更优异的耐腐蚀性能。阻抗|Z|与频率的关系曲线表明,阻抗的变化规律与Nyquist图的变化规律相同(图8b),表明Mo51Fe34B15非晶合金丝材的阻抗性能确实比Mo51Co17Fe17B15非晶合金丝材优异。值得注意的是,这两种非晶合金丝材催化剂的阻抗随着频率的提高而降低,表明高频率加速其腐蚀失效。
图8
图8
Mo51Co17Fe17B15和Mo51Fe34B15非晶合金在结晶紫溶液中的极化曲线和奈奎斯特图
Fig.8
Electrochemical behavior of Mo51Co17Fe17B15 and Mo51Fe34B15 amorphous alloys in Crystal Violet solutions (a) polarization curve, (b) Nyquist plots derived from EIS measurements, illustration: Impedance frequency diagram. (room temperature, natural pH, 20 × 10-6 crystal violet solution, 0.1 mol/L H2O2)
2.3.2 降解前后非晶合金丝材的表面形貌和元素
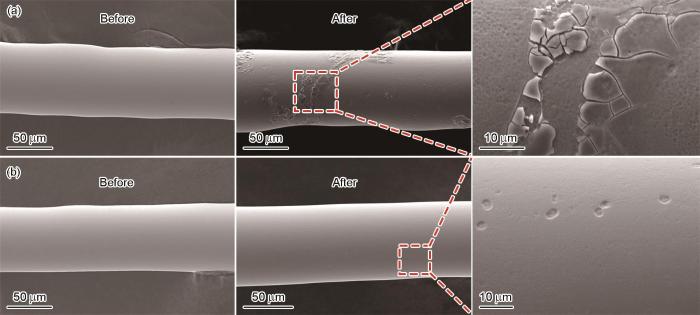
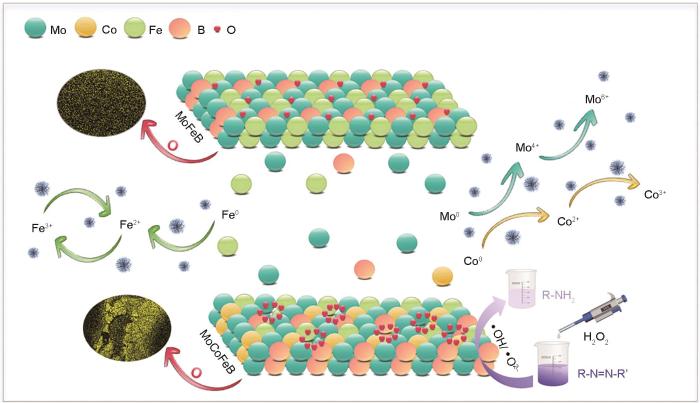
因为降解有机物废水的反应主要发生在固-液界面,分析降解反应后非晶合金丝材表面可揭示其反应过程。图9分别给出了Mo51Co17Fe17B15和Mo51Fe34B15非晶合金丝材降解偶氮染料结晶紫前后的表面形貌。可以看出,原始非晶合金的表面光滑,但是随着降解反应的进行其表面出现了腐蚀形貌。如图9a所示,Mo51Co17Fe17B15表面出现明显的腐蚀层,在降解反应过程中这些腐蚀层逐渐剥落,露出新的非晶合金基底层。过快的腐蚀使非晶合金来不及发生降解催化反应部分表面就消耗了。从图9b可见Mo51Fe34B15丝材典型的选择性腐蚀坑,除了少量的腐蚀坑,其表面保持着光滑。其原因是,Mo51Fe34B15非晶合金表面元素的均匀分布降低了生成局部电池效应和腐蚀集中的可能性,有助于活性位点的均匀分布而使催化效率提高。与Mo51Co17Fe17B15非晶合金的腐蚀行为不同,Mo51Fe34B15非晶合金更利于参与降解催化反应而提高对染料废水的降解性能。
图9
图9
Mo51Co17Fe17B15和Mo51Fe34B15非晶合金丝材降解偶氮染料废水前后的表面形貌
Fig.9
Surface morphologies of Mo51Co17Fe17B15 (a) and Mo51Fe34B15 (b) amorphous alloy wires before and after degradation of azo dye wastewater (Room temperature, natural pH, 20 × 10-6 crystal violet solution, 0.1 mol/L H2O2)
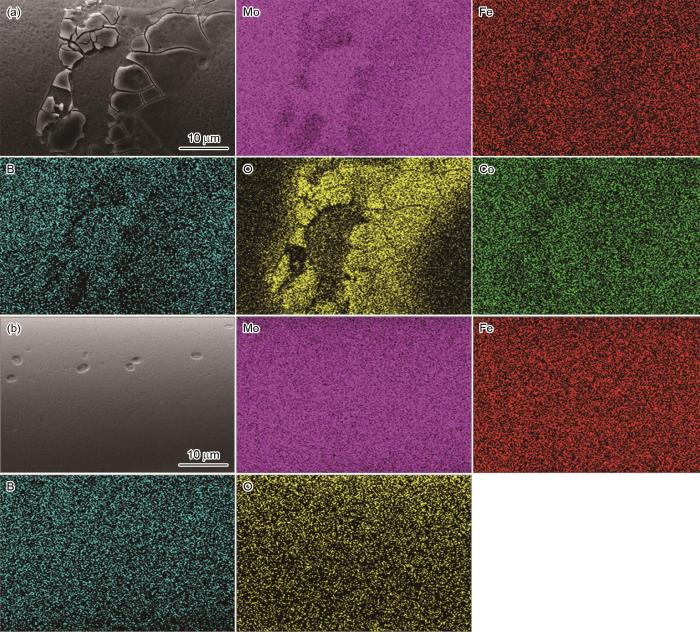
用EDS分析了Mo51Co17Fe17B15和Mo51Fe34B15非晶合金丝材表面元素的分布,以揭示非晶合金降解染料废水的机理。从图10a可见,在Mo51Co17Fe17B15非晶合金中的腐蚀产物处Mo元素明显溃泛,Co和Fe元素的变化趋势相同。同时,腐蚀产物处明显的O元素富集表明发生了严重的氧化。非晶合金表面的氧化层隔离了内部还原性零价金属与染料溶液的接触,阻止后续降解反应而使反应速率降低[32]。从图10b可见,Mo51Fe34B15非晶合金表面均匀分布的元素提供了均匀的活性位点,有助于提高其催化效率和耐腐蚀性。其原因是,元素均匀分布减少了产生局部电池效应和腐蚀集中的可能性,也是Mo51Fe34B15非晶合金丝材比Mo51Co17Fe17B15催化降解性能更加优异的重要原因。
图10
图10
Mo51Co17Fe17B15和Mo51Fe34B15非晶合金丝材降解染料废水后的表面EDS能谱
Fig.10
Surface EDS spectra of Mo51Co17Fe17B15 (a) and Mo51Fe34B15 (b) amorphous alloy wires after degradation of dye wastewater (Room temperature, natural pH, 20 × 10-6 crystal violet solution, 0.1 mol/L H2O2)
2.3.3 非晶合金丝材降解偶氮染料后表面元素的价态
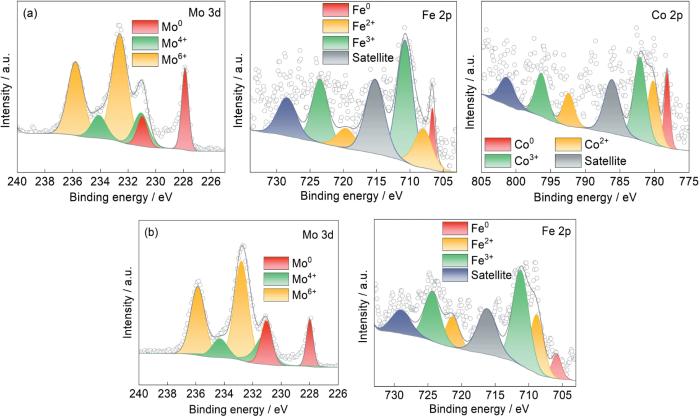
为了分析Mo51Co17Fe17B15和Mo51Fe34B15非晶合金催化剂的降解机理,用XPS分析了降解反应后其表面金属元素化学价态的变化,结果如图11所示。图11a分别给出了Mo51Co17Fe17B15非晶合金中Mo 3d、Fe 2p和Co 2p的图谱,图11b给出了对应的Mo51Fe34B15非晶合金中的Mo 3d和Fe 2p图谱。可以看出,降解反应后两个样品中还含有大量的Mo0、Co0、Fe0,其中Mo51Co17Fe17B15非晶合金除了腐蚀的裂纹区域零价金属较少外,腐蚀坑中心的腐蚀产物已经完全剥落,暴露出富含零价金属的基体。Mo51Fe34B15非晶合金降解反应后,表面剩余大量的零价金属,因为其没有参与降解反应。在Mo51Co17-Fe17B15和Mo51Fe34B15两种非晶合金的Mo 3d图谱中,位于227.9 eV和230.9 eV的拟合峰对应于Mo0,位于231.0 eV和234.1 eV的两峰对应于Mo4+,另外两个位于232.6 eV和235.8 eV的拟合峰对应于Mo6+。这表明,在降解过程中Mo0仍然是电子的主要贡献者,氧化生成了MoO2和MoO3,峰的面积表明MoO3的占比例最大。图11a中Co 2p的图谱表明,非晶合金表面的Co0演变成Co2+和Co3+,其主要腐蚀产物为Co3O4。
图11
图11
Mo51Co17Fe17B15和Mo51Fe34B15降解偶氮染料废水后非晶合金丝材表面的XPS谱
Fig.11
Surface XPS results of amorphous alloy wires after degradation of azo dye wastewater by Mo51Co17Fe17B15 (a) and Mo51Fe34B15 (b) (Room temperature, natural pH, 20 × 10-6 crystal violet solution, 0.1 mol/L H2O2)
Mo51Co17Fe17B15和Mo51Fe34B15两种非晶合金的Fe 2p图谱都有Fe0、Fe2+和Fe3+,对各峰面积的计算结果表明Mo51Co17Fe17B15和Mo51Fe34B15非晶合金中Fe2+的相对含量分别为23.3%、34%。随着降解反应的进行,Mo元素和Co元素始终维持着高Mo6+、高Co3+和高Fe3+。高价态离子较难跃迁到低价态,极大的限制了活性物质的产生和速率的提高。这表明,催化剂表面维持低价态金属元素有利于为降解提供大量的电子转移基础以加快降解催化反应[33]。Fe的氧化还原电位(E0 = -0.44 V)比Co (E0 = -0.28 V)低,更能促进H2O2分解。铁的二价态更容易跃迁到三价态,使铁在芬顿反应中更稳定和有效。在芬顿反应中,Co3+的稳定性较高,而Fe3+可与H2O2和H+反应还原为Fe2+,从而维持循环反应促进废水降解。这表明,Mo51Fe34B15非晶合金表面更高的Fe2+含量表明其电子转移更快,这也是其对偶氮染料溶液降解效率较高的关键原因。同时,降解反应后非晶催化剂中的B元素通常以B-O键的形式存在,而O元素则主要存在于金属氧化物和有机C化物中[19]。
根据上述对降解结晶紫染料溶液后Mo51Co17Fe17B15和Mo51Fe34B15非晶合金丝材的元素分布、表面形貌、微观结构和电子结构的分析,在图12中给出其催化降解机理示意图。浸入结晶紫溶液中的非晶合金丝材,其中的金属元素氧化产生自由电子。这些自由电子在非晶合金丝材表面形成电子云,从而使其表面电子结构发生变化。在降解过程中,原始非晶合金样品中的Mo0、Co0和Fe0分别为降解反应提供4、2和2个电子。从Mo4+到Mo6+、Co2+到Co3+和Fe2+到Fe3+的氧化,是Fenton-like/Fenton变化中产生
图12
图12
Mo51Co17Fe17B15和Mo51Fe34B15非晶合金丝材降解结晶紫染料废水的机理示意图
Fig.12
Schematic diagram of degradation mechanism of crystal violet dye wastewater by Mo51Co17Fe17B15 and Mo51Fe34B15 amorphous alloy wires
3 结论
(1) 用熔融纺丝法制备的Mo51Co17Fe17B15和Mo51Fe34B15两种新型MoFeB系非晶合金丝材,具有优异热稳定性。
(2) Mo51Fe34B15非晶合金催化剂对结晶紫溶液的降解效率比Mo51Co17Fe17B15非晶合金的高。Mo51Co17Fe17B15和Mo51Fe34B15非晶合金降解催化剂具有宽泛的pH值适应性。
(3) Mo51Fe34B15非晶合金丝材优异的耐腐蚀性能和表面更高的Fe2+含量有利于为催化降解提供大量的电子转移基础,适当的腐蚀行为和更高的电子转移能力是其具有优异降解性能的关键原因。
参考文献
An over review on recently developed techniques, mechanisms and intermediate involved in the advanced azo dye degradation for industrial applications
[J].
A critical review of the aniline transformation fate in azo dye wastewater treatment
[J].
Preparation of acid-alkali modified coal fly ash adsorbent and its removal performance on dyes
[J].
酸-碱改性粉煤灰吸附剂的制备及其对染料的去除性能
[J].
Synthesis of N-doped hierarchical porous carbon and its adsorption capacity for acid orange 74
[J].N-doped hierarchical porous carbon (HPCT) was synthesized by adjusting the final activation temperature, with indole as carbon and nitrogen source, CaO as template coupled with KOH activation, and then the adsorption performance of acid orange 74 on HPCT was investigated. BET results show that the surface area of HPCT increases with the increase of activation temperature. The specific surface area of the as-made HPC900 is up to 1629 m2/g when the final activation temperature was 900℃. The FESEM and TEM results demonstrate that the HPCT has the interconnected layer structure. With the rising activation temperature the wall width of HPCT becomes thinner. XPS results show that nitrogen functional groups existed on the HPCT surface, the content of C-NH2 increases gradually as temperature rose. The above functional group is conducive to the π-π stacking effect and electrostatic interaction with absorbate acid orange 74, which is beneficial to the adsorption process. The adsorption isotherm results indicate that the adsorption process could be described by Freundlich model, the equilibrium adsorption capacity of which was more than 270 mg/g by the equilibrium concentration of 50 mg/L. The kinetic results show that the pseudo first-order kinetic equation can better describe the adsorption process, while the physical adsorption is the rate-control step.
吲哚基掺氮分级多孔炭的制备及其对酸性橙74的吸附性能
[J].以吲哚为碳源、氧化钙为模板耦合KOH活化并调节活化终温,制备出表面掺氮的层状分级多孔炭(HPC<sub>T</sub>),研究了其对酸性橙74的吸附性能。结果表明:随着活化温度的提高这种多孔炭的比表面积增大,活化终温为900℃时制得的HPC<sub>900</sub>比表面积高达1629 m<sup>2</sup>/g。这种炭材料具有相互连接的层状结构,且随着活化温度的提高炭壁层变薄。这种炭材料的表面有丰富的含氮官能团C-NH<sub>2</sub>,随着活化温度的提高C-NH<sub>2</sub>的含量随之提高。C-NH<sub>2</sub>官能团与酸性橙74发生π-π堆积效应或静电相互作用,有利于提高其吸附性能。Freundlich模型能很好地描述HPC<sub>T</sub>对染料的吸附过程,在50 mg/L的平衡浓度下HPC<sub>900</sub>对废水中酸性橙74的吸附量超过270 mg/g;拟一级动力学方程能更好的描述HPC<sub>T</sub>对酸性橙74的吸附过程,物理吸附为控速步骤。
Preparation of carboxylic acid grafted starch adsorption resin and its dye removal performance
[J].Carboxylic acid grafted starch adsorption resin (CSR) was synthesized by using natural starch (RS) as matrix, acrylic acid (AA) and vinyl acetate (VAc) as raw materials in the presence of ammonium persulfate (APS) and sodium bisulfite (SHS). The resulted CSR product was characterized by SEM, IR, XRD, 13C-NMR and GPC. The results show that when the initiator concentration was 0.03 mol/L, the monomer mass ratio n (AA): n (VAc) was 3:1 and the monomer concentration was 0.8 mol/l, the prepared CSR product presents the carboxyl group content of 19.26% with adsorption capacity of 17.35mg/g for malachite green, in other words, the water resistance and chemical stability and the tolerance to acid, alkali and enzyme of CSR resin were enhanced in comparison to those of the natural starch. After alkali treatment the adsorption capacity of the resin can be further improved. Linear molecules in CSR resin gradually transformed into branched type, resulting in complex network structure of macromolecules. The molecular mass of the main chain of CSR decreased with the increase of monomer dosage and AA / VAc ratio. The adsorption capacity of CSR for basic fuchsin (BF), methylene blue (MB) and malachite green (MG) is better than that of 001×7 strong acid ion exchange resin, D151 weak acid ion exchange resin, carboxymethyl cellulose CMC and carboxymethyl starch CMS and other synthetic and natural polymer adsorbents. The CSR has broad-spectrum of adsorption closed to that of active carbon. The zerocharge point pHpzc of CSR was 3.83, which was much lower than that of natural starch resin (pHpzc=7.38), that may be an important reason for the increase of adsorption capacity of cationic dyes. The CSR has good dye adsorption performance in a wide range of pH values, and the adsorption capacity reaches the maximum value when pH=8.5. The decolorization rate of CSR for mixed dye waste water was 87.42%. Finally the CSR had strong regeneration ability. Even after eight regeneration cycles, its decolorization rate was not lower than 88.6% of the initial adsorption ability.
羧酸型接枝淀粉吸附树脂的制备和对染料的去除性能
[J].
Fabrication of composite material based on MOFs and its adsorption properties for methylene blue dyes
[J].Zr-based metal-organic frameworks (MOFs) UIO-66-NH2 was modified with methacrylate anhydride, and then the modified MOFs were deposited onto polypropylene (PP) nonwoven fabric while real-time curing assisted by UV irradiation to prepare composite of MOFS/nonwoven fabric (PSP). The changes of crystal form and morphology of UIO-66-NH2 before and after modification were analyzed, and the binding fastness of MOFs (UIO-66-NH2-MET) to PP nonwovens was examined, and the effect of adsorption conditions on the adsorption properties of PSP composite was investigated. The results show that UIO-66-NH2-MET could be prepared through modifying the UIO-66-NH2 structure with methacrylate anhydride group. After modification, the crystal structure of MOFs remained unchanged and the original frame structure was retained. UIO-66-NH2-MET has good fastness on PP nonwovens. No mass loss occurred after repeated washing, and the PSP composite retained good adsorption effect. The optimum conditions for PSP composites to adsorb methylene blue (MB) are as follows: The initial concentration of the dye was 50 mg/L, the adsorption time was 300 min, and the pH value was 9.
基于MOFs的复合材料制备及其对亚甲基蓝染料的吸附性能
[J].用甲基丙烯酸酐对锆基MOFs UiO-66-NH<sub>2</sub>进行改性,然后用紫外光固化将改性后的MOFs UiO-66-NH<sub>2</sub>负载于聚丙烯(PP)非织造布制备出MOFs与非织造布(PSP)复合材料。分析了改性前后UiO-66-NH<sub>2</sub>的晶型和形貌变化并测试了MOFs(UiO-66-NH<sub>2</sub>-MET)与PP非织造布的结合牢度,测试了吸附条件对PSP复合材料吸附性能的影响。结果表明:通过在UiO-66-NH<sub>2</sub>结构中修饰甲基丙烯酸酐基团制备出UiO-66-NH<sub>2</sub>-MET,改性后MOFs的晶型结构没有变化,保留了原有的框架结构;UiO-66-NH<sub>2</sub>-MET与PP非织造布间有良好的结合牢度。PSP复合材料经历多次水洗后未发生质量损失,保留了良好的吸附效果;PSP复合材料吸附亚甲基蓝(MB)的最佳条件为:染料的初始浓度为50 mg/L,吸附时间为300 min,pH值为9。
A review of catalytic performance of metallic glasses in wastewater treatment: Recent progress and prospects
[J].
Application of Fe-based amorphous alloy in industrial wastewater treatment: a review
[J].
Rapid degradation of azo dye by Fe-based metallic glass powder
[J].
The pivotal role of boron in improving the azo dye degradation of glassy Fe-based catalysts
[J].
Crystallization kinetics of amorphous alloys Fe73.5Si13.5- x Ge x B9Cu1Nb3 (x = 3, 6)
[J].
Fe73.5Si13.5- x Ge x B9Cu1Nb3 (x = 3, 6)非晶合金的结晶动力学
[J].用标准单辊甩带技术在大气环境下制备Fe<sub>73.5</sub>Si<sub>13.5-</sub><sub>x</sub>Ge<sub>x</sub>B<sub>9</sub>Cu<sub>1</sub>Nb<sub>3</sub>(x=3, 6)非晶条带, 分别在470℃、510℃、550℃和590℃对非晶条带进行真空等温退火1 h后, 在非晶基体中形成了纳米晶相。用X射线衍射(XRD), 透射电镜(TEM)和差示扫描量热法(DSC)测量研究了快淬态和热处理后样品的结构和结晶动力学。基于差热分析的数据, 使用Kissinger, Ozawa和Augis-Bennett模型计算了非晶条带的结晶激活能, 利用Johnson-Mehl-Avrami(JMA)方程计算了非晶条带初始结晶的局域Avrami因子n。局域Avrami因子n随晶化体积分数α的显著变化说明, 非晶条带非等温初始结晶的机理在不同的晶化阶段是不同的。晶化初期的机理是扩散控制的三维形核和晶粒生长的整体晶化, 形核速率逐渐减小; 晶化中后期为一维形核和生长的表面晶化过程, 形核速率近似为零。基于XRD和TEM测量结果, 分别在510℃、550℃和590℃真空等温退火1 h后, 在Fe<sub>73.5</sub>Si<sub>13.5-</sub><sub>x</sub>Ge<sub>x</sub>B<sub>9</sub>Cu<sub>1</sub>Nb<sub>3</sub>(x=3, 6)非晶条带中析出的α-Fe (Si, Ge)相的平均晶粒尺寸D小于15 nm。
Influence of Co and Ni on invar effect of (Fe71.2B24Y4.8)96Nb4 bulk metallic glass
[J].
Co和Ni对(Fe71.2B24Y4.8)96Nb4块体非晶合金因瓦效应的影响
[J].进行了[(Fe<sub>100-</sub><sub>x</sub>Co<sub>x</sub>)<sub>71.2</sub>B<sub>24</sub>Y<sub>4.8</sub>]<sub>96</sub>Nb<sub>4</sub>(x=0~60)和[(Fe<sub>100-</sub><sub>x</sub>Ni<sub>x</sub>)<sub>71.2</sub>B<sub>24</sub>Y<sub>4.8</sub>]<sub>96</sub>Nb<sub>4</sub>(x=0~20)块体非晶合金的热膨胀实验。结果表明,加入Co和Ni使合金的因瓦效应减弱,替代量x相同时减弱的程度也基本相同。这些结果说明,添加Co和Ni对Fe原子局域结构的主要影响是减少了Fe-Fe原子对的数目。同时还发现,居里温度与磁性相变结束后的热膨胀系数呈反向变化,与结构弛豫导致的自由体积释放有关。
Fabrication of nano-porous Co by dealloying for supercapacitor and azo-dye degradation
[J].Nano-porous Co was prepared by electrochemically dealloying of Zr56Al16Co28 amorphous alloy in 0.5%(mass fraction) NH4F and 1 mol/L (NH4)2SO4 mixed solution. Nano-porous Co has the bicontinuous porous structure with large specific surface area and fast charge transfer ability. The prepared nano-porous Co exhibits good performance in many aspects: firstly, it delivers a high specific capacitance of 318 F/g at 2 A/g, suggesting its good performance as supercapacitor electrode; secondly, it exhibits degradation efficiencies under square wave potential as high as 96% for Direct Blue 6 and Acid Orange II respectively. The degradation ability of nano-porous Co electrode per unit mass is 3.5 times higher than that of Zr56Al16Co28 amorphous alloy electrode.
脱合金制备纳米多孔Co及其超级电容器和对偶氮染料的降解性能
[J].
Preparation of magnetic amino acid-functionalized aluminum alginate gel polymer and its super adsorption on azo dyes
[J].A novel magnetic amino acid functionalized aluminum alginate gel polymer Gly/Al/SA@Fe3O4 was prepared via droplet polymerization technique with sodium alginate (SA) as raw material, which was crosslinked with Al(Ⅲ) ions, while glycine (Gly) and Fe3O4 were simultaneously added. The prepared Gly/Al/SA@Fe3O4 was characterized by means of scanning electron microscopy (SEM), X-ray energy dispersive spectroscopy (EDS), Fourier infrared spectroscopy (FT-IR), X-ray diffractometer (XRD) and vibrating sample magnetometer (VSM). Its absorption performance for azo dyes was also investigated. The results show that Gly/Al/SA@Fe3O4 is a kind of three-dimensional network-like polymer particles with a fancy fold structure on the surface, and its magnetic response ability is good. Gly/Al/SA@Fe3O4 shows strong adsorption performance with high adsorption rate for Direct Black 19(DB 19) and Direct Brown 2(DB 2) dyes in water. The dynamic equilibrium adsorption capacities reached 2500 mg/L and 3126 mg/L, respectively for the above two dyes in 15min and 60 min. The adsorption process can be described by a quasi-second-order rate equation, and the isothermal adsorption data conform to Langmuir model. The interaction between the adsorbents and dye molecules was synergistic through electrostatic adsorption, hydrogen bonding, ligand exchange and chemisorption. Gly/Al/SA@Fe3O4 particles are green and environment-friendly, and have strong water purification ability for wastewater containing high concentration azo dye, therefore, it can be used for rapid solid-liquid separation with magnetic field.
磁性氨基酸功能化海藻酸铝凝胶聚合物的制备及对偶氮染料的超强吸附
[J].以海藻酸钠为原料,采用液滴聚合法将其与Al(Ⅲ)离子交联并引入甘氨酸和Fe<sub>3</sub>O<sub>4</sub>,制备出磁性氨基酸功能化海藻酸铝凝胶聚合物(Gly/Al/SA@Fe<sub>3</sub>O<sub>4</sub>),使用扫描电镜(SEM)、X射线能谱仪(EDS)、傅里叶红外光谱仪(FT-IR)、X射线衍射仪(XRD)和振动样品磁强计(VSM)等手段对其表征,研究了这种凝胶聚合物对偶氮染料的吸附性能。结果表明,Gly/Al/SA@Fe<sub>3</sub>O<sub>4</sub>是一种表面具有花式褶皱结构的三维网状聚合物颗粒,其磁响应能力良好。Gly/Al/SA@Fe<sub>3</sub>O<sub>4</sub>对水体中直接黑19(DB 19)和直接棕2(DB 2)染料的吸附性能超强,吸附速率极高,吸附15 min和60 min达到动态平衡的吸附量分别为2500和3126 mg/L。吸附过程可用拟二级速率方程描述,等温吸附数据符合Langmuir模型。吸附剂与染料分子间的相互作用通过静电吸附、氢键作用、配体交换和化学吸附协同实现。Gly/Al/SA@Fe<sub>3</sub>O<sub>4</sub>颗粒绿色环保,对高浓度偶氮染料废水有超强的净水性能,并可用磁场进行快速固液分离。
Preparation of electrode materials of amorphous Co-W-B/Carbon cloth composite and their electro-catalytic performance for electrolysis of water
[J].Amorphous Co-W-B was deposited on carbon cloth (CC) to fabricate a self-supported Co-W-B/CC composite electrode by using chemical reduction method. Electrochemical analysis show that Co-W-B/CC materials exhibited excellent electrocatalytic performance for electrolysis of water in 1 mol/L NaOH solution. Among others, the Co-50W-B/CC (the ratio of [WO42-]/([WO42-]+[Co2+]) is 50% in the synthesis process) shows the best electrocatalytic activity, i.e. for the Co-50W-B/CC catalyst, when the low overpotential is 0.394 V by ampere density of 10mA/cm2, the corresponding Tafel slope is 96.8 mV/dec for the oxygen evolution reaction (OER), whilst when the overpotential is 0.098 V by ampere density of -10 mA/cm2, the corresponding Tafel slope is 117.4 mV/dec for the hydrogen evolution reaction (HER). EIS analysis result implies that the Co-50W-B/CC possesses nearly the same catalytic activity as the noble metal-based materials at low current density, which can mainly be attributed to both the high intrinsic catalytic activity and the large electrochemical active area.
非晶Co-W-B/碳布复合电极材料的制备及其电解水催化性能
[J].通过湿化学还原在碳布(CC)表面沉积非晶Co-W-B催化活性物质,制备一种自支撑Co-W-B/碳布(Co-W-B/CC)复合电极材料。电化学研究结果表明,Co-W-B/CC材料在NaOH溶液(1 mol/L)中表现出良好的电解水催化性能。制备过程中[WO<sub>4</sub><sup>2-</sup>]/([WO<sub>4</sub><sup>2-</sup>]+[Co<sup>2+</sup>])比值为50%的Co-50W-B/CC样品其催化活性最高:10 mA/cm<sup>2</sup>时的OER过电位为0.394V,OER过程的Tafel斜率为96.8 mV/dec;-10 mA/cm<sup>2</sup>时的HER过电位为0.098 V,HER过程的Tafel斜率为117.4 mV/dec。对电化学阻抗的分析结果表明,本征催化活性和电化学活性面积两者的提高,使Co-50W-B/CC样品在较低的电流密度下具有与贵金属基材料相近的催化活性。
Electrocatalytic oxygen evolution performance of high entropy FeCoNiMoCr alloy thin film electrode
[J].Thin film of high entropy FeCoNiMoCr alloy was deposited on Ti substrate by magnetron sputtering method to obtain high entropy film electrode. The surface morphology, composition, phase constituent, structure and performance of the electrode were characterized by means of surface profilometer, SEM-EDS, XRD and electrochemical workstation. The results show that the electrode surface is rough, the constituent elements are evenly distributed, the film thickness is about 2.40 μm, and the film is amorphous. The electrode showed good oxygen evolution performance and good stability in the alkaline solution. Under the condition of current density of 10.0 mA/cm2, the overpotential was 360 mV, the Tafel slope was 73.45 mV/dec. Under the condition of overpotential of 360 mV, the current density was not significantly attenuated after continuous use for 24 hours. The results of cyclic voltammetry and electrochemical impedance analysis show that due to the improved intrinsic catalytic activity, the film electrode have electrocatalytic oxygen evolution performance better than that of the noble metal oxide RuO2 (over potential 409 mV, Tafel slope 94.18 mV/dec).
FeCoNiMoCr高熵合金薄膜电极的电催化析氧性能
[J].用磁控溅射法在Ti基底上沉积了FeCoNiMoCr高熵合金薄膜并制成电极,用SEM和EDS观察和分析了电极表面和横截面的形貌和元素分布,用表面轮廓测量仪测量了电极的表面粗糙度,用XRD分析了电极的物相和结构,使用电化学工作站表征了电极的电化学性能。结果表明,电极的表面粗糙、元素分布均匀,电极上的膜厚约为2.40 μm,薄膜呈非晶态。电极在碱性溶液中表现出良好的析氧性能和稳定性。在电流密度为10.0 mA/cm<sup>2</sup>条件下,过电位为360 mV、Tafel斜率为73.45 mV/dec。在过电位为360 mV的条件下连续使用24 h,电流密度没有明显的衰减。循环伏安实验和电化学阻抗分析的结果表明,FeCoNiMoCr高熵合金薄膜本征催化活性的提高使电极的电催化析氧性能优于贵金属RuO<sub>2</sub>(过电位为409 mV,Tafel斜率为94.18 mV/dec)。
Excellent capability in degrading azo dyes by MgZn-based metallic glass powders
[J].
Surface aging behaviour of Fe-based amorphous alloys as catalysts during heterogeneous photo Fenton-like process for water treatment
[J].
Rapid degradation of direct blue dye by Co-based amorphous alloy wire
[J].
MoCoB metallic glass microwire catalysts for highly efficient and pH-universal degradation of wastewater
[J].
Application of Fe-based metallic glasses in wastewater treatment
[J].
Decolorization of azo dye solution by Fe-Mo-Si-B amorphous alloy
[J].
Efficient degradation of rhodamine B using Fe-based metallic glass catalyst by Fenton-like process
[J].An efficient heterogeneous catalyst, Fe-based metallic glass (Fe–Si–B amorphous ribbon), was successfully prepared for Fenton-like degradation of rhodamine B (RhB) by a melt-spinning method. The catalyst was characterized using XRD and SEM. The effects of various reaction parameters such as H2O2 dosage, temperature, initial pH value, Fe–Si–B dosage and initial RhB concentration on the degradation of RhB were studied. Almost complete degradation of RhB (20 mg L−1) was achieved within only 10 min by 0.5 g L−1 Fe–Si–B catalyst and 1.6 mM H2O2 at pH 3.0 at 295 K. Kinetic analyses showed that the degradation process could be described by a pseudo-first-order kinetic model. The catalytic stability was also investigated and it was found that the Fe–Si–B catalyst exhibited good structural stability and no loss of performance even after three cycles. It was concluded that the Fe–Si–B amorphous ribbon was a potential heterogeneous Fenton-like catalyst for industrial wastewater treatment.
Unexpected high performance of Fe-based nanocrystallized ribbons for azo dye decomposi-tion
[J].
Chemically dealloyed MgCuGd metallic glass with enhanced catalytic activity in degradation of phenol
[J].
Effect of Co addition on catalytic activity of FePCCu amorphous alloy for methylene blue degradation
[J].
Cu-based metallic glass with robust activity and sustainability for wastewater treatment
[J].
Under what conditions can a glass be formed?
[J].
Catalytic oxidation of phenol in wastewater — A new application of the amorphous Fe78Si9B13 alloy
[J].
Highly efficient and stable CuZr-based metallic glassy catalysts for azo dye degradation
[J].Metallic glasses with the unique disordered atomic structure and metastable nature have been recently applied to degrade the azo dyes and other organic pollutants based on their superior catalytic performance. In this work, the functional properties of six CuZr-based metallic glassy ribbons with the different nominal components in degrading Acid Orange Ⅱ (AO Ⅱ) azo dyes were investigated. The Cu47.5Zr46Al6.5 metallic glassy ribbons could exhibit the more advanced catalytic performance for degradation process, which could completely degrade azo dye aqueous solution within 30 min. Additionally, the Cu47.5Zr46Al6.5 metallic glassy ribbons also showed the excellent cyclic stability along with approximately 97.68 % degradation efficiency after 10 cycles. These excellent catalytic performance and stability are closely related to the synergistic effect of exposed copper nanoparticles and produced copper oxides in the reaction, which contributes to accelerate the generation of more hydroxyl radicals (·OH) to react with dye molecules. Our findings can be able to develop a novel potential metallic glassy material for the functional application of wastewater treatment.
Decolorization of azo dye solution by Fe-Mo-Si-B amorphous alloy
[J].
Effects of the addition of Co, Ni or Cr on the decolorization properties of Fe-Si-B amorphous all-oys
[J].
Three-dimensional hierarchical porous structures of metallic glass/copper composite catalysts by 3D printing for efficient wastewater treatments
[J].