制备Al2O3/Fe-Al复合阻氚涂层的方法,主要有包埋渗铝+原位氧化、热浸铝+原位氧化、电镀沉积+原位氧化等[16~18]。包埋渗铝本质上是一种原位化学气相沉积(CVD),操作简单,可用于制备各种尺寸和复杂几何形状的涂层组件[19,20]。铝化物涂层的制备涉及复杂的化学反应,许多研究人员研究了不同制备过程中的热力学[21~24]。对于铝化物涂层的制备,目前尚存在一些争议。Majumdar等[24]使用NH4Cl作为催化剂在450℃~1050℃的宽温度范围内研究了SS316不锈钢在包埋渗铝过程中铝化物的生成。结果表明,在450℃~650℃的较低温度范围内Fe2Al5相的生长动力学比其他铁铝化物高得多,并且在渗铝试样中生成了由Fe2Al5相组成的单层。在高于700℃的温度,从外表面向基体生成了由Fe2Al5/FeAl/Fe(Al)组成的多个铁铝化物层。Dong等[25]使用AlCl3作为催化剂,在不同温度(600、650和680℃)和对316L不锈钢进行了不同时间(2、3和4 h)的低温渗铝。结果表明,先生成的是Fe2Al5相,其次是FeAl和FeAl3相,温度降至422℃以下Fe3Al相才开始生成。动力学研究结果表明,低温渗铝过程的活化能较低(164.78 kJ/mol)。Kobayashi等[26]研究了低碳钢经热浸法渗铝后在873~1323 K内表面Fe-Al金属间化合物层的生长机理。结果表明,在873~923 K扩散Fe2Al5主要在试样表面生成,高温度于1273 K时优先生成FeAl和Fe3Al相层。生长FeAl和Fe3Al层所需的活化能,分别为QFeAl = 180 kJ/mol和Q
1 实验方法
1.1 实验用材料
实验用材料有316L不锈钢和渗剂。
316L不锈钢的化学成分,列于表1。用自动精密切割机(MECA TOME T210)将材料切割成尺寸为30 mm × 25 mm × 1 mm的矩形薄片,将其依次用120#、320#、600#碳化硅砂纸磨去表面氧化层,然后置于丙酮溶液中超声清洗10 min,吹干后备用。
表1 316L不锈钢合金的成分(质量分数,%)
Table 1
| Element | Cr | Ni | Mo | Mn | Si | C | Fe |
|---|---|---|---|---|---|---|---|
| Content | 16.0~18.0 | 10.0~14.0 | 2.0~3.0 | ≤2.0 | ≤1.0 | ≤0.03 | Bal. |
渗剂的配比为8%Al + 1%NH4Cl + 91%Al2O3。将渗剂混合研磨均匀后装入刚玉坩埚(ϕ45 mm × 50 mm)中,将试样放置于坩埚中间位置后使用高温胶水(回天2767系列)密封,在常温固化24 h后放入马弗炉中依次在80℃保温2 h、在150℃保温2 h,以使高温胶水进一步固化。
1.2 渗铝
将固化后的坩埚置于真空管式炉内,以4℃/min速率升温至预定温度后保温预定时间,然后随炉冷却至室温,取出样品洗净烘干并称其质量。实验温度和保温时间列于表2。
表2 包埋渗铝实验方案
Table 2
| Sample | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Temperature / oC | 600 | 650 | 700 | 750 | 750 | 750 | 750 | 750 | 800 | 850 | 900 |
| Time / h | 5 | 5 | 5 | 1 | 3 | 5 | 7 | 10 | 5 | 5 | 5 |
1.3 微观结构的表征
用型号为DM6000的金相显微镜(OM)观察试样渗铝后的表面形貌;用型号为D8 Advance的X射线衍射仪(XRD)测定Fe-Al渗层的物相组成,2θ角的范围为10°~90°,扫描步长和滞留时间分别为0.02°和0.5 s;用附带能谱仪的LEO 1530VP型扫描电子显微镜(SEM)分析Fe-Al渗层截面的形貌和成分。
2 实验结果
2.1 渗铝温度对Fe-Al渗层的影响
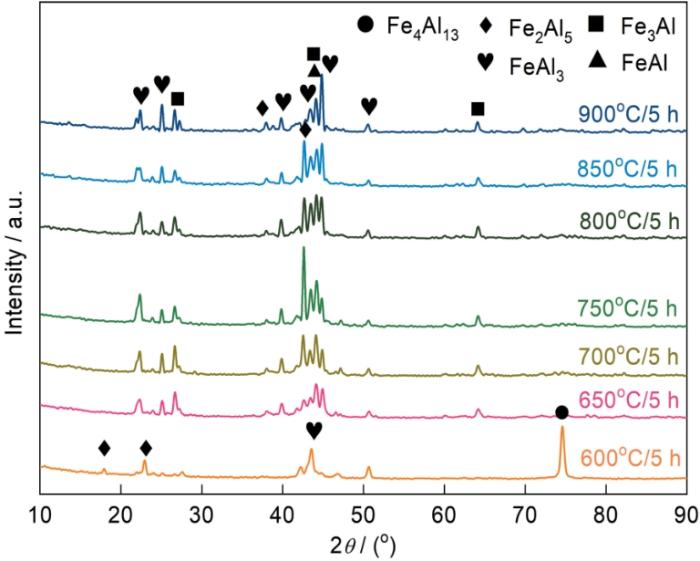
图1为试样分别在600℃~900℃渗铝温度范围内渗铝5 h后在试样表面所测得的XRD图谱。可以看到在不同温度范围内,渗层的物相主要由FeAl3、Fe2Al5、FeAl、Fe3Al等物相组成。值得注意的是,在600℃时,可以观测到较强的316L基体的相峰和FeAl3相的相峰,当温度升高时,基体的相峰消失;这也侧面表明,600℃时试样表面所形成的渗层很薄,XRD射线穿透至基体;而随着温度升高,Al元素的进一步向基体内部扩散,渗层厚度增加,基体的衍射峰不再带出。
图1
图1
在不同渗铝温度制备的Fe-Al渗层的XRD谱
Fig.1
XRD patterns of Fe-Al aluminized layer prepared at different aluminizing temperatures
此外,根据衍射峰的相关高度,可以发现随着渗铝温度的升高Fe2Al5相的相峰呈现先增高后降低的趋势,在750℃时峰值达到最高;而FeAl和Fe3Al等相的相峰随温度升高而升高。初步判断在温度高于750℃时,Fe2Al5相会加快和Fe原子的反应速度形成FeAl相,从而导致Fe2Al5相的相峰高度下降。
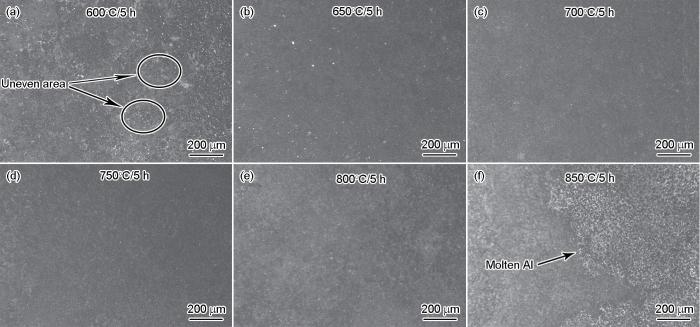
图2
图2
在不同渗铝温度制备的试样的表面组织形貌金相照片
Fig.2
Metallographic photos of the surface morphology of the sample at different aluminizing temperatures
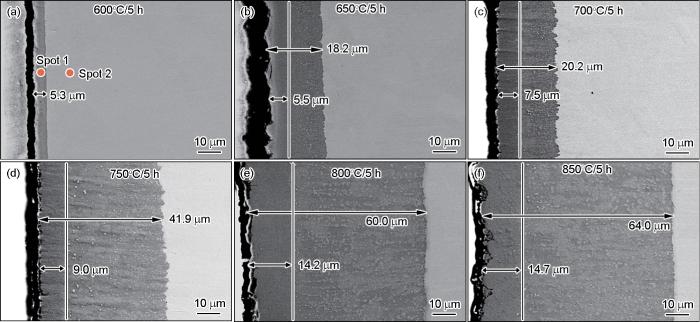
图3
图3
在不同渗铝温度制备的渗层的截面形貌SEM照片
Fig.3
SEM photos of the cross-section morphology of the aluminized layer at different aluminizing temperatures
此外,在不同渗铝温度下,渗层和基体连接紧密,内部无明显空洞存在。值得注意的是,在650℃~750℃渗铝温度范围内,与基底相邻的渗层形成了明显的连续紧凑的锯齿状结构,如图3b~d所示;特别地,750℃时其形貌特征较之更为明显,在这种形貌特征下,渗层和基体形成冶金结合,界面之间的结合力得到加强,而这与渗层内部物相的晶体结构有关。通过XRD和EDS分析(图5)可知,渗层内部为具有斜方点阵结构的Fe2Al5相,Al原子占据在其C轴结点处,因该轴上空位较多,因从Al原子可以沿该轴向基体快速扩散,最终使渗层呈现出锯齿状形貌特征嵌入基体界面[27]。伴随温度升高,齿状特征逐渐变宽乃至消失,同时渗层的表面质量也逐渐变差,如图3e,f所示。
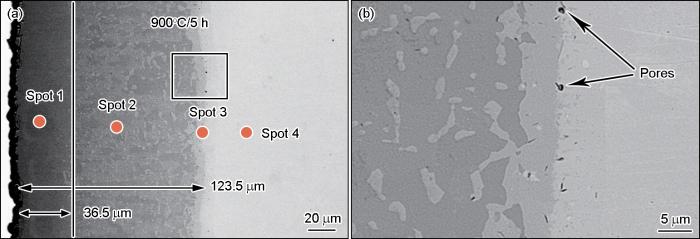
图4
图4
在900℃制备的渗层截面形貌的SEM照片
Fig.4
SEM photos of the cross-section morphology of the aluminized layer at 900oC
图5
图5
不同渗铝时间制备的Fe-Al渗层的XRD谱
Fig.5
XRD patterns of Fe-Al aluminized layer prepared at different aluminizing time
为了进一步确认渗层的物相组成和形成规律,分别对在600℃和900℃渗铝5 h所得渗层的截面进行了EDS分析。在渗层的不同位置对其进行了点扫描,扫描位置如图3a和图4a所示,所得元素的成分组成列于表3。根据各点对应的Al、Fe原子分数,在600℃渗层极薄,在点1处的点扫描得到Al和Fe的原子比近似等于3∶1。结合XRD结果可推断,其物相为FeAl3相;在900℃渗层较厚且呈多层结构,分别对3个位置进行了点扫描。从外向内Al含量逐渐降低而Fe含量逐渐提高,根据其原子之比推断出点1、2、3处的物相依次为FeAl3、Fe2Al5和FeAl相,点4处则为基体。这表明,不同的渗铝温度,Al、Fe原子的扩散的结果渗层最先形成的是最外层的FeAl3相,其次是中间层的Fe2Al5相和内层FeAl相。这个结论,与现有文献的结果一致[25,27,28]。
表3 对图3a和图4a中各点元素成分的扫描
Table 3
| Temperature / oC | Spot | Elements / %, atom fraction | |||
|---|---|---|---|---|---|
| Al | Fe | Cr | Ni | ||
| 600 | 1 | 56.12 | 19.80 | 19.46 | 4.62 |
| 2 | 0 | 59.06 | 18.92 | 10.76 | |
| 900 | 1 | 60.04 | 20.62 | 5.29 | 2.50 |
| 2 | 53.01 | 23.62 | 5.63 | 6.63 | |
| 3 | 40.94 | 31.27 | 8.57 | 9.17 | |
| 4 | 0 | 56.12 | 14.57 | 10.56 | |
2.2 渗铝时间对Fe-Al渗层的影响
在750℃渗铝不影响基材性能,得到的渗层厚度适中,渗层与基体呈冶金结合,因此在750℃渗铝研究渗铝时间对Fe-Al渗层的影响。
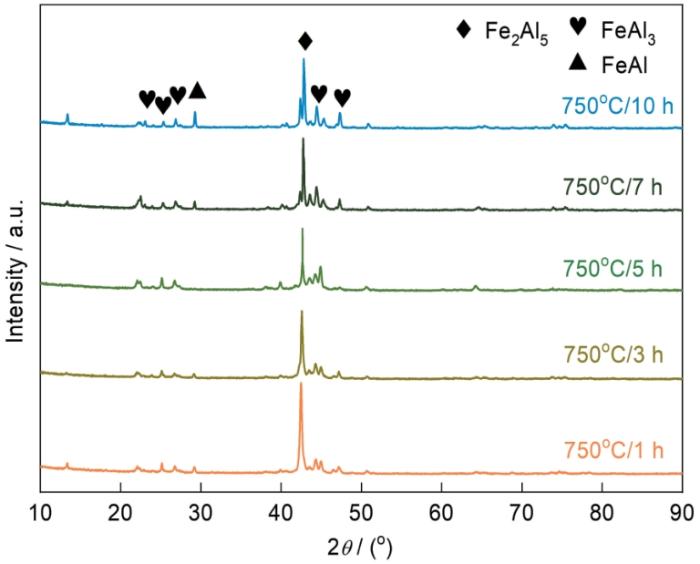
图5给出了在750℃渗铝1 h、3 h、5 h、7 h、10 h试样表面的XRD谱。可以看到,渗铝不同时间得到的Fe-Al渗层,其物相组成没有明显的变化,都是由Fe2Al5、FeAl3等相组成。这表明,渗铝时间对Fe-Al渗层的物相组成的影响较小。
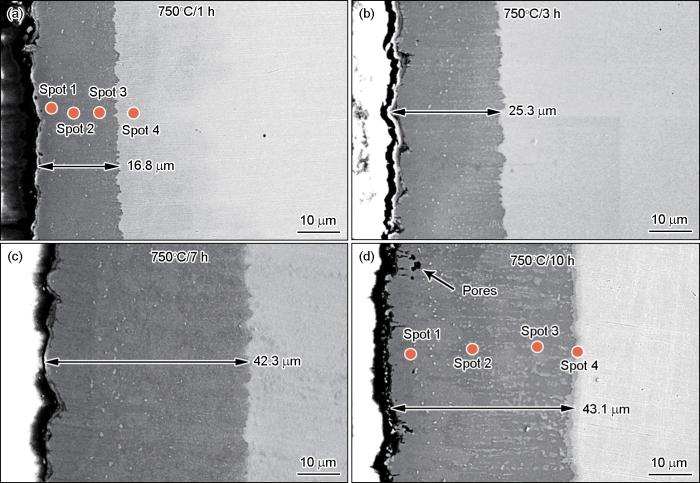
图6
图6
不同渗铝时间制备的渗层截面形貌的SEM照片和EDS点扫描位置
Fig.6
SEM photos of the cross-section morphology of the aluminized layer under different aluminizing time and the point scanning position of the aluminized layer
表4 对应图6a,d中各点扫描的元素成分组成
Table 4
| Time / h | Spot | Elements / %, atom fraction | |||
|---|---|---|---|---|---|
| Al | Fe | Cr | Ni | ||
| 1 | 1 | 64.77 | 20.72 | 7.62 | 6.90 |
| 2 | 60.91 | 26.35 | 7.90 | 4.84 | |
| 3 | 57.00 | 25.68 | 11.43 | 5.89 | |
| 4 | 0.64 | 66.21 | 21.00 | 12.14 | |
| 10 | 1 | 63.96 | 22.04 | 6.91 | 7.08 |
| 2 | 59.77 | 31.78 | 8.39 | 0.06 | |
| 3 | 55.16 | 20.38 | 24.17 | 0.29 | |
| 4 | 13.22 | 56.36 | 21.45 | 9.03 | |
3 讨论
3.1 渗层的热力学分析
所得Fe(Al)各相的吉布斯自由能ΔG与温度的关系,如图7所示。
图7
图7
Fe-Al化合物的吉布斯能ΔG随温度T的变化
Fig.7
Variations of Gibbs energy ΔG with the temperature T for Fe-Al compounds
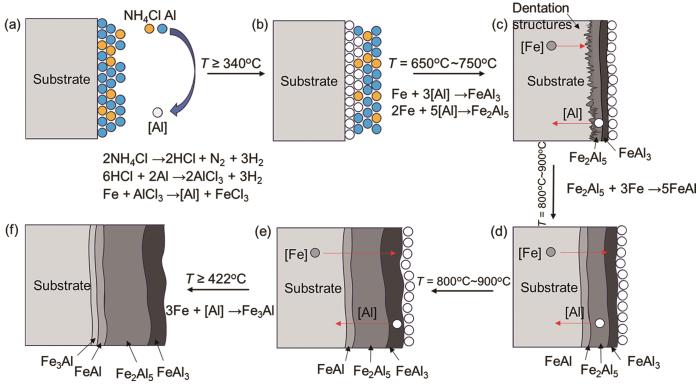
在600℃~900℃反应式(10~12)的吉布斯自由能ΔG随着温度的升高而增大,但是Fe2Al5、FeAl和FeAl3相的吉布斯自由能仍小于0,且Fe2Al5相的自由能最低。这表明,随着Al和Fe元素的固态扩散Fe2Al5相最容易生成。尽管XRD测试分析结果表明在反应初期FeAl3相已经在基体表面生成,但是Fe2Al5相生成后其生长速率远高于FeAl3相。随着Fe2Al5相的生长FeAl3相成为Fe2Al5相生长所需的铝源。这表明,FeAl3相的生长受到了抑制并被消耗,使其逐渐分散在表层。最后,渗层以Fe2Al5相为主,而FeAl3相只在其表面。同时,在温度高于695 K (422℃)反应(13)的吉布斯能ΔG变为正值,表明只有温度降低到422℃以下ΔG < 0时Fe3Al相才能生成。
图8
图8
多层相结构的铁铝合金渗层的形成过程模型
Fig.8
Schematic diagram of the formation process model of Fe-Al alloy aluminized layer with multilayer phase structure
3.2 渗层动力学
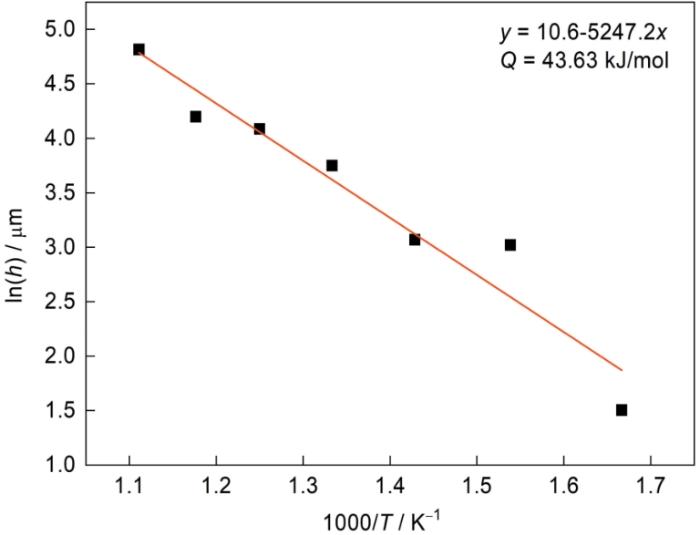
如上所述,渗层内以Fe2Al5相为主,因此根据渗层厚度可大致计算出Al原子在该体系中的扩散激活能和生长动力学方程。
图9给出了渗层厚度h的对数ln(h)与温度T的倒数1000/T值之间的关系,二者之间较强的线性关系表明,温度对渗层生长速率(kp)的影响符合Arrhenius规律,即
式中Q为扩散激活能,kJ/mol;R为气体常数,取8.314;T为绝对温度,K;A1 为常数[27]。
图9
图9
渗层厚度与渗铝温度的关系
Fig.9
Relation curve between aluminizing temperature and aluminizing layer thickness
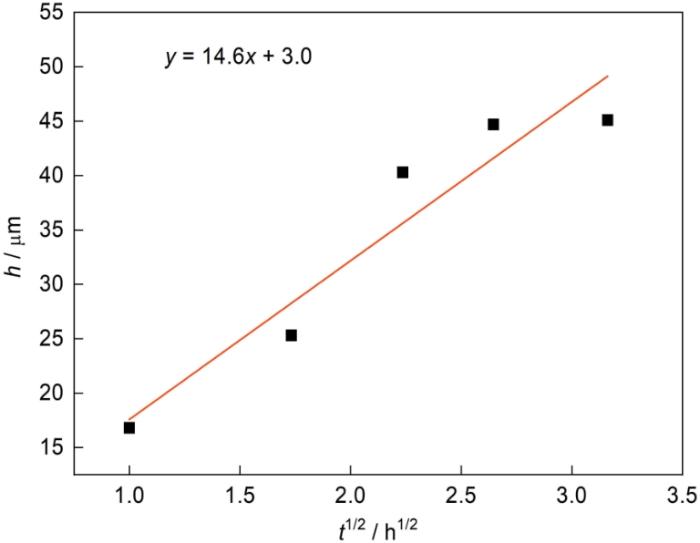
同时,在750℃进行不同时间的渗铝,得到了不同厚度的渗层。根据以上结果并结合现有文献,可构建渗层厚度h与渗铝时间t1/2的关系,如图10所示。结果表明,随着渗铝时间的增加渗层的厚度呈现抛物线规律。根据拟合结果,渗铝不同时间得到的Fe-Al渗层的生长动力学方程为
图10
图10
渗层厚度与渗铝时间之间的关系
Fig.10
Relation curve between aluminizing time and thickness of aluminized layer
综上所述,渗铝温度和时间对渗层物相受的影响较小,对相的含量和渗层厚度的影响较大。提高渗铝温度和延长渗铝时间均能提高Fe2Al5相的含量和增加渗层厚度,且渗铝温度对渗层的影响大于渗铝时间对渗层的影响。
4 结论
(1) 随着渗铝温度的提高和渗铝时间的延长,渗层的厚度随之增加且呈多层结构。渗铝温度和时间对渗层的物相组成影响不大,渗层都主要由FeAl3相和Fe2Al5相组成;FeAl3相最先在基体表面生成,Fe2Al5相的极快生长抑制了FeAl3相的生长;只有当渗铝温度低于422℃而Fe3Al相的吉布斯自由能小于0时,FeAl3相才开始生成。
(2) 渗铝温度为650℃~750℃时渗层呈锯齿状结构嵌入基底,与具有斜方点阵晶体结构的Fe2Al5相相关;温度升至800℃~900℃渗层的齿状形貌特征逐渐消失且渗层的表面质量降低,在Fe(Al)各相之间的界面处出现孔隙。
(3) 渗铝温度对渗层生长速率(kp)的影响符合Arrhenius公式规律,渗铝温度范围为600℃~900℃时Al原子的扩散激活能约为43.63 kJ/mol;单据Arrhenius公式拟合出不同渗铝时间Fe-Al渗层的生长动力学方程式h = 14.6 t1/2 + 3.0。
参考文献
Preparation of Cr2O3 film by MOCVD as hydrogen permeation barrier
[J].
Current progress of tritium fuel cycle technology for CFETR
[J].
Tritium permeation behavior through pyrolytic carbon in tritium production using high-temperature gas-cooled reactor for fusion reactors
[J].
Nanostructured thin films for hydrogen-permeation barrier
[J]. J.
Tritium permeation experiments using reduced activation ferritic/martensitic steel tube and erbium oxide coating
[J].
Hydrogen interactions with intrinsic point defects in hydrogen permeation barrier of α-Al2O3: a first-principles study
[J].
Fabrication of Al2O3/FeAl coating as tritium permeation barrier on tritium operating component on quasi-CFETR scale
[J].
Formation of Al2O3/FeAl coatings on a 9Cr-1Mo steel, and corrosion evaluation in flowing Pb-17Li loop
[J].
Growth of inter-metallic compound layers on CLAM steel by HDA and preparation of permeation barrier by oxidation
[J].
Mechanical and tribological properties of crystalline aluminum nitride coatings deposited on stainless steel by magnetron sputtering
[J].
Performances of AlN coatings as hydrogen isotopes permeation barriers
[J].
An effective low-temperature strategy for sealing plasma sprayed Al2O3-based coatings
[J].
Preparation technique and alloying effect of aluminide coatings as tritium permeation barriers: A review
[J].
A deuterium permeation barrier by hot-dipping aluminizing on AISI 321 steel
[J].
Characterization of the alumina film with cerium doped on the iron-aluminide diffusion coating
[J].
An Al2O3/Ni-Al tritium permeation barrier coating for the potential application in thorium-based molten salt reactor
[J].
Research progress on formation mechanism and low temperature preparation technology of Al2O3 film on surface of Fe-Al/Al2O3 tritium permeation barrier
[J].
Fe-Al/Al2O3阻氚涂层表面Al2O3薄膜形成机制与低温制备技术的研究进展
[J].
Bonding properties of Fe/Al2O3 ceramic gradient coating
[J].
Fe/Al2O3陶瓷梯度涂层的结合性能
[J].
Preparation of α-Al2O3/NiAl multilayer coatings on GH3535 superalloy surface by pack cementation and subsequent in situ oxidation
[J].
Design and properties of Fe-Al/Al2O3/TiO2 composite tritium-resistant coating prepared through pack cementation and sol-gel method
[J].
Iron aluminide coatings on ferritic steels by CVD-FBR technology
[J].
Effects of reactive gaseous mixture and time on the growth rate and composition of aluminium diffusion coatings by CVD-FBR on 12Cr-ferritic steel
[J].
Formation of aluminide coatings on ferritic-martensitic steels by a low-temperature pack cementation process
[J].
Formation of Al2O3/Fe-Al layers on SS316 surface by pack aluminizing and heat treatment
[J].
Formation mechanism of multilayer aluminide coating on 316L stainless steel by low-temperature pack cementation
[J].
Control of intermetallic compound layers at interface between steel and aluminum by diffusion-treatment
[J].
Preparation method and diffusion mechanism of Fe-Al coating on Q235 low carbon steel by pack aluminizing
[J].The Fe-Al coating, with compactness, stiffness, and continuity, could be prepared on Q235 low carbon steel by pack aluminizing. The phase structure, morphology, composition, and hardness of the prepared coating were characterized by XRD, SEM, EDS, and micro-hardness tester respectively. Results indicate that the Fe-Al coating is composed of Fe2Al5 and FeAl3 phases, whilst, the coating fabricated at 750℃ is particularly rich in Fe2Al5 phase. With the rising temperature, the thickness of Fe-Al coating increases, whereas the micro-hardness decreases. As a result of aluminizing for different time, the formed coatings are composed of the two phases Fe2Al5 and FeAl3 as well. However, with the increasing aluminizing time, the content of FeAl3 phase decreases, while the micro-hardness of the coating decreases slightly. Finally, a diffusion mechanism related with the formation of Fe-Al coating is proposed based on the comprehensive analysis on the thermodynamics and kinetics of pack aluminizing process.
包埋渗铝法制备 Fe-Al渗层及其扩散机制
[J].包埋渗铝法可在钢基体表面制备出一层致密、坚固、连续的Fe-Al渗层,以改善基体性能。本文在不同温度和不同时间下对Q235低碳钢进行包埋渗铝,形成Fe-Al渗层,采用X射线衍射、扫描电镜及能谱分析等方法研究了渗铝层的物相结构、表面及截面形貌和成分,采用显微硬度仪测量了截面硬度。结果表明,不同渗铝温度下获得的渗铝层,主要含有Fe<sub>2</sub>Al<sub>5</sub>和FeAl<sub>3</sub>两相,且750℃得到的渗层存在较多Fe<sub>2</sub>Al<sub>5</sub>相;随着渗铝温度升高,Fe-Al渗层厚度增加,Al原子扩散系数增大,但显微硬度降低;不同渗铝时间下制备的渗铝层,物相仍以Fe<sub>2</sub>Al<sub>5</sub>和FeAl<sub>3</sub>为主,但随着渗铝时间延长,FeAl<sub>3</sub>含量减少,且Al原子扩散系数变大,渗层显微硬度略有降低。在进一步分析Fe-Al渗层形成的热力学与动力学基础上,总结了渗铝层形成的扩散机制。
Formation and phase transformation of aluminide coating prepared by low-temperature aluminizing process
[J].
Pack aluminisation of low alloy steels at te-mperatures below 700oC
[J].
Low temperature pack aluminising kinetics of nickel electroplated on creep resistant ferritic steel
[J].
Microstructure, growth kinetics and mechanical properties of interface layer for roll bonded aluminum-steel clad sheet annealed under argon gas protection
[J].