S-TiO2/UiO-66-NH2 composite for boosted photocatalytic Cr(VI) reduction and bisphenol A degradation under LED visible light
1
2020
... 在电镀、纺织、制药和皮革等行业,Cr(VI)浓度过高的废水危害人体健康、动植物生长和污染环境[1,2].化学沉淀、离子交换和吸附等传统的废水处理方法易产生二次污染、循环性差且能耗过高[3~5].与传统的方法相比,光催化技术的能耗低、效率高且不易产生二次污染[6]. ...
Designing porous nickel architectures for adsorptive removal of Cr(VI) to achieve drinking water standard
1
2020
... 在电镀、纺织、制药和皮革等行业,Cr(VI)浓度过高的废水危害人体健康、动植物生长和污染环境[1,2].化学沉淀、离子交换和吸附等传统的废水处理方法易产生二次污染、循环性差且能耗过高[3~5].与传统的方法相比,光催化技术的能耗低、效率高且不易产生二次污染[6]. ...
Controllable fabrication of visible-light-driven CoS x /CdS photocatalysts with direct Z-scheme heterojunctions for photocatalytic Cr(VI) reduction with high efficiency
1
2020
... 在电镀、纺织、制药和皮革等行业,Cr(VI)浓度过高的废水危害人体健康、动植物生长和污染环境[1,2].化学沉淀、离子交换和吸附等传统的废水处理方法易产生二次污染、循环性差且能耗过高[3~5].与传统的方法相比,光催化技术的能耗低、效率高且不易产生二次污染[6]. ...
Br-doping of g-C3N4 towards enhanced photocatalytic performance in Cr(VI) reduction
1
2020
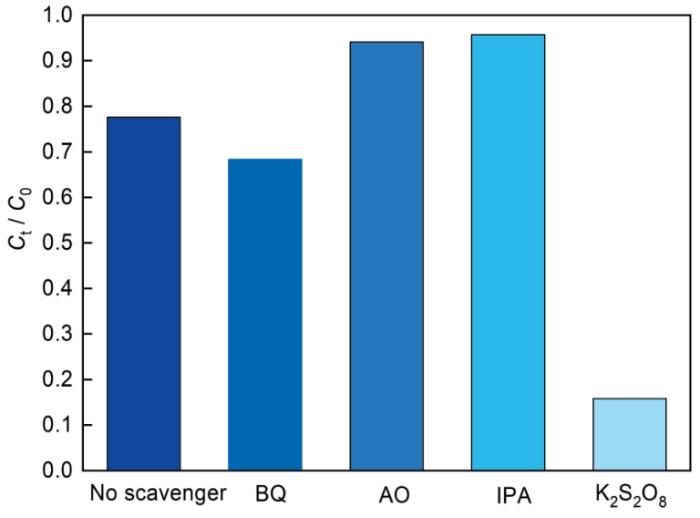
... 分别用AO、BQ、IPA和K2S2O8作为h+、·O、·OH和e-的捕获剂,测定了光催化反应过程中的活性物种[45].从图9可见,K2S2O8的加入大大降低了Ag3PO4/MIL-125(Ti)-2对Cr(VI)的光催化还原率,说明光生e-在Cr(VI)的还原过程中有决定性作用.加入BQ时Cr(VI)的还原率有所下降,其原因可能是O2/·O介导的还原反应中电子传递降低[4].与之相反的是,加入AO和IPA后Cr(VI)的还原率显著提高,因为光生h+被捕获后与光生e-的分离效率提高,参与还原Cr(VI)的光生e-更多,而·OH被捕获后限制了Cr(III)再次被氧化为Cr(VI). ...
In-situ preparation of NH2-MIL-125(Ti)/BiOCl composite with accelerating charge carriers for boosting visible light photocatalytic activity
1
2019
... 在电镀、纺织、制药和皮革等行业,Cr(VI)浓度过高的废水危害人体健康、动植物生长和污染环境[1,2].化学沉淀、离子交换和吸附等传统的废水处理方法易产生二次污染、循环性差且能耗过高[3~5].与传统的方法相比,光催化技术的能耗低、效率高且不易产生二次污染[6]. ...
Preparation and visible light catalytic performance of g-C3N4/POPs heterojunction
1
2023
... 在电镀、纺织、制药和皮革等行业,Cr(VI)浓度过高的废水危害人体健康、动植物生长和污染环境[1,2].化学沉淀、离子交换和吸附等传统的废水处理方法易产生二次污染、循环性差且能耗过高[3~5].与传统的方法相比,光催化技术的能耗低、效率高且不易产生二次污染[6]. ...
g-C3N4/POPs异质结制备及其可见光催化性能
1
2023
... 在电镀、纺织、制药和皮革等行业,Cr(VI)浓度过高的废水危害人体健康、动植物生长和污染环境[1,2].化学沉淀、离子交换和吸附等传统的废水处理方法易产生二次污染、循环性差且能耗过高[3~5].与传统的方法相比,光催化技术的能耗低、效率高且不易产生二次污染[6]. ...
MnO2 nanosheets supported metal-organic framework MIL-125(Ti) towards efficient visible light photocatalysis: Kinetic and mechanistic study
1
2020
... 金属有机骨架结构(Metal organic frameworks, MOFs)材料,由金属簇或无机金属离子通过配位键连接而成.MOFs材料具有较大的比表面积、多种金属中心和易于调整的形貌[7,8],可用于光催化领域[9~11].MIL-125(Ti)是一种制备成本低、易于获取的经典MOFs材料,但是较宽的带隙能限制其在光催化领域的应用[12~14].制备复合材料和染料敏化等方式,可提高其光能利用率.Li等[15]用溶剂热法制备了一种以TiO2为壳、以MIL-125(Ti)为芯的核壳结构复合材料,具有良好的光催化还原性能.Hu等[16]制备了MIL-125(Ti)/ZnIn2S4复合材料,Ti4+与Ti3+之间的界面电荷转移和协同作用可高效去除RhB和Cr(VI).Han等[17]通过RhB敏化MIL-125(Ti),光照60 min对甲基橙的降解效率达到90%.Ag3PO4是一种窄带隙(2.45 eV)半导体材料,在可见光区具有光催化活性.Ag3PO4的价带能级较低和导带中有π*轨道,这种特殊的能带结构使其氧化能力和电子迁移速率提高,具有高效降解水体中污染物的性能[18].但是,在光催化过程中Ag3PO4易被光腐蚀,使其光催化效率和循环利用率降低[19,20].将MOFs与Ag3PO4结合制备的复合材料,如Ag/Ag3PO4/HKUST-1[21]、Ag3PO4/MIL-101/NiFe2O4[22]、Ag3PO4/超薄MOF纳米片[23]等,具有优异的光催化性能和稳定性.鉴于此,本文用原位沉积将Ag3PO4纳米颗粒沉积在MIL-125(Ti)上制备复合光催化剂Ag3PO4/MIL-125(Ti),研究其光催化还原Cr(VI)的性能. ...
Fabrication of composite material based on MOFs and its adsorption properties for methylene blue dyes
1
2021
... 金属有机骨架结构(Metal organic frameworks, MOFs)材料,由金属簇或无机金属离子通过配位键连接而成.MOFs材料具有较大的比表面积、多种金属中心和易于调整的形貌[7,8],可用于光催化领域[9~11].MIL-125(Ti)是一种制备成本低、易于获取的经典MOFs材料,但是较宽的带隙能限制其在光催化领域的应用[12~14].制备复合材料和染料敏化等方式,可提高其光能利用率.Li等[15]用溶剂热法制备了一种以TiO2为壳、以MIL-125(Ti)为芯的核壳结构复合材料,具有良好的光催化还原性能.Hu等[16]制备了MIL-125(Ti)/ZnIn2S4复合材料,Ti4+与Ti3+之间的界面电荷转移和协同作用可高效去除RhB和Cr(VI).Han等[17]通过RhB敏化MIL-125(Ti),光照60 min对甲基橙的降解效率达到90%.Ag3PO4是一种窄带隙(2.45 eV)半导体材料,在可见光区具有光催化活性.Ag3PO4的价带能级较低和导带中有π*轨道,这种特殊的能带结构使其氧化能力和电子迁移速率提高,具有高效降解水体中污染物的性能[18].但是,在光催化过程中Ag3PO4易被光腐蚀,使其光催化效率和循环利用率降低[19,20].将MOFs与Ag3PO4结合制备的复合材料,如Ag/Ag3PO4/HKUST-1[21]、Ag3PO4/MIL-101/NiFe2O4[22]、Ag3PO4/超薄MOF纳米片[23]等,具有优异的光催化性能和稳定性.鉴于此,本文用原位沉积将Ag3PO4纳米颗粒沉积在MIL-125(Ti)上制备复合光催化剂Ag3PO4/MIL-125(Ti),研究其光催化还原Cr(VI)的性能. ...
基于MOFs的复合材料制备及其对亚甲基蓝染料的吸附性能
1
2021
... 金属有机骨架结构(Metal organic frameworks, MOFs)材料,由金属簇或无机金属离子通过配位键连接而成.MOFs材料具有较大的比表面积、多种金属中心和易于调整的形貌[7,8],可用于光催化领域[9~11].MIL-125(Ti)是一种制备成本低、易于获取的经典MOFs材料,但是较宽的带隙能限制其在光催化领域的应用[12~14].制备复合材料和染料敏化等方式,可提高其光能利用率.Li等[15]用溶剂热法制备了一种以TiO2为壳、以MIL-125(Ti)为芯的核壳结构复合材料,具有良好的光催化还原性能.Hu等[16]制备了MIL-125(Ti)/ZnIn2S4复合材料,Ti4+与Ti3+之间的界面电荷转移和协同作用可高效去除RhB和Cr(VI).Han等[17]通过RhB敏化MIL-125(Ti),光照60 min对甲基橙的降解效率达到90%.Ag3PO4是一种窄带隙(2.45 eV)半导体材料,在可见光区具有光催化活性.Ag3PO4的价带能级较低和导带中有π*轨道,这种特殊的能带结构使其氧化能力和电子迁移速率提高,具有高效降解水体中污染物的性能[18].但是,在光催化过程中Ag3PO4易被光腐蚀,使其光催化效率和循环利用率降低[19,20].将MOFs与Ag3PO4结合制备的复合材料,如Ag/Ag3PO4/HKUST-1[21]、Ag3PO4/MIL-101/NiFe2O4[22]、Ag3PO4/超薄MOF纳米片[23]等,具有优异的光催化性能和稳定性.鉴于此,本文用原位沉积将Ag3PO4纳米颗粒沉积在MIL-125(Ti)上制备复合光催化剂Ag3PO4/MIL-125(Ti),研究其光催化还原Cr(VI)的性能. ...
Recent advances in strategies to modify MIL-125(Ti) and its environmental applications
1
2021
... 金属有机骨架结构(Metal organic frameworks, MOFs)材料,由金属簇或无机金属离子通过配位键连接而成.MOFs材料具有较大的比表面积、多种金属中心和易于调整的形貌[7,8],可用于光催化领域[9~11].MIL-125(Ti)是一种制备成本低、易于获取的经典MOFs材料,但是较宽的带隙能限制其在光催化领域的应用[12~14].制备复合材料和染料敏化等方式,可提高其光能利用率.Li等[15]用溶剂热法制备了一种以TiO2为壳、以MIL-125(Ti)为芯的核壳结构复合材料,具有良好的光催化还原性能.Hu等[16]制备了MIL-125(Ti)/ZnIn2S4复合材料,Ti4+与Ti3+之间的界面电荷转移和协同作用可高效去除RhB和Cr(VI).Han等[17]通过RhB敏化MIL-125(Ti),光照60 min对甲基橙的降解效率达到90%.Ag3PO4是一种窄带隙(2.45 eV)半导体材料,在可见光区具有光催化活性.Ag3PO4的价带能级较低和导带中有π*轨道,这种特殊的能带结构使其氧化能力和电子迁移速率提高,具有高效降解水体中污染物的性能[18].但是,在光催化过程中Ag3PO4易被光腐蚀,使其光催化效率和循环利用率降低[19,20].将MOFs与Ag3PO4结合制备的复合材料,如Ag/Ag3PO4/HKUST-1[21]、Ag3PO4/MIL-101/NiFe2O4[22]、Ag3PO4/超薄MOF纳米片[23]等,具有优异的光催化性能和稳定性.鉴于此,本文用原位沉积将Ag3PO4纳米颗粒沉积在MIL-125(Ti)上制备复合光催化剂Ag3PO4/MIL-125(Ti),研究其光催化还原Cr(VI)的性能. ...
Visible-light-driven photocatalytic degradation of pollutants over Cu-doped NH2-MIL-125(Ti)
0
2018
In-situ implantation of plasmonic Ag into metal-organic frameworks for constructing efficient Ag/NH2-MIL-125/TiO2 photoanode
1
2020
... 金属有机骨架结构(Metal organic frameworks, MOFs)材料,由金属簇或无机金属离子通过配位键连接而成.MOFs材料具有较大的比表面积、多种金属中心和易于调整的形貌[7,8],可用于光催化领域[9~11].MIL-125(Ti)是一种制备成本低、易于获取的经典MOFs材料,但是较宽的带隙能限制其在光催化领域的应用[12~14].制备复合材料和染料敏化等方式,可提高其光能利用率.Li等[15]用溶剂热法制备了一种以TiO2为壳、以MIL-125(Ti)为芯的核壳结构复合材料,具有良好的光催化还原性能.Hu等[16]制备了MIL-125(Ti)/ZnIn2S4复合材料,Ti4+与Ti3+之间的界面电荷转移和协同作用可高效去除RhB和Cr(VI).Han等[17]通过RhB敏化MIL-125(Ti),光照60 min对甲基橙的降解效率达到90%.Ag3PO4是一种窄带隙(2.45 eV)半导体材料,在可见光区具有光催化活性.Ag3PO4的价带能级较低和导带中有π*轨道,这种特殊的能带结构使其氧化能力和电子迁移速率提高,具有高效降解水体中污染物的性能[18].但是,在光催化过程中Ag3PO4易被光腐蚀,使其光催化效率和循环利用率降低[19,20].将MOFs与Ag3PO4结合制备的复合材料,如Ag/Ag3PO4/HKUST-1[21]、Ag3PO4/MIL-101/NiFe2O4[22]、Ag3PO4/超薄MOF纳米片[23]等,具有优异的光催化性能和稳定性.鉴于此,本文用原位沉积将Ag3PO4纳米颗粒沉积在MIL-125(Ti)上制备复合光催化剂Ag3PO4/MIL-125(Ti),研究其光催化还原Cr(VI)的性能. ...
Hybrid three MOFs composites (ZIF-67@ZIF-8@MIL-125-NH2): Enhancement the biological and visible-light photocatalytic activity
1
2020
... 金属有机骨架结构(Metal organic frameworks, MOFs)材料,由金属簇或无机金属离子通过配位键连接而成.MOFs材料具有较大的比表面积、多种金属中心和易于调整的形貌[7,8],可用于光催化领域[9~11].MIL-125(Ti)是一种制备成本低、易于获取的经典MOFs材料,但是较宽的带隙能限制其在光催化领域的应用[12~14].制备复合材料和染料敏化等方式,可提高其光能利用率.Li等[15]用溶剂热法制备了一种以TiO2为壳、以MIL-125(Ti)为芯的核壳结构复合材料,具有良好的光催化还原性能.Hu等[16]制备了MIL-125(Ti)/ZnIn2S4复合材料,Ti4+与Ti3+之间的界面电荷转移和协同作用可高效去除RhB和Cr(VI).Han等[17]通过RhB敏化MIL-125(Ti),光照60 min对甲基橙的降解效率达到90%.Ag3PO4是一种窄带隙(2.45 eV)半导体材料,在可见光区具有光催化活性.Ag3PO4的价带能级较低和导带中有π*轨道,这种特殊的能带结构使其氧化能力和电子迁移速率提高,具有高效降解水体中污染物的性能[18].但是,在光催化过程中Ag3PO4易被光腐蚀,使其光催化效率和循环利用率降低[19,20].将MOFs与Ag3PO4结合制备的复合材料,如Ag/Ag3PO4/HKUST-1[21]、Ag3PO4/MIL-101/NiFe2O4[22]、Ag3PO4/超薄MOF纳米片[23]等,具有优异的光催化性能和稳定性.鉴于此,本文用原位沉积将Ag3PO4纳米颗粒沉积在MIL-125(Ti)上制备复合光催化剂Ag3PO4/MIL-125(Ti),研究其光催化还原Cr(VI)的性能. ...
Efficient immobilization of enzymes on amino functionalized MIL-125-NH2 metal organic framework
0
2022
Synthesis of Bi2WO6@NH2-MIL-125(Ti): A S-scheme photocatalyst with enhanced visible light catalytic activity
1
2020
... 金属有机骨架结构(Metal organic frameworks, MOFs)材料,由金属簇或无机金属离子通过配位键连接而成.MOFs材料具有较大的比表面积、多种金属中心和易于调整的形貌[7,8],可用于光催化领域[9~11].MIL-125(Ti)是一种制备成本低、易于获取的经典MOFs材料,但是较宽的带隙能限制其在光催化领域的应用[12~14].制备复合材料和染料敏化等方式,可提高其光能利用率.Li等[15]用溶剂热法制备了一种以TiO2为壳、以MIL-125(Ti)为芯的核壳结构复合材料,具有良好的光催化还原性能.Hu等[16]制备了MIL-125(Ti)/ZnIn2S4复合材料,Ti4+与Ti3+之间的界面电荷转移和协同作用可高效去除RhB和Cr(VI).Han等[17]通过RhB敏化MIL-125(Ti),光照60 min对甲基橙的降解效率达到90%.Ag3PO4是一种窄带隙(2.45 eV)半导体材料,在可见光区具有光催化活性.Ag3PO4的价带能级较低和导带中有π*轨道,这种特殊的能带结构使其氧化能力和电子迁移速率提高,具有高效降解水体中污染物的性能[18].但是,在光催化过程中Ag3PO4易被光腐蚀,使其光催化效率和循环利用率降低[19,20].将MOFs与Ag3PO4结合制备的复合材料,如Ag/Ag3PO4/HKUST-1[21]、Ag3PO4/MIL-101/NiFe2O4[22]、Ag3PO4/超薄MOF纳米片[23]等,具有优异的光催化性能和稳定性.鉴于此,本文用原位沉积将Ag3PO4纳米颗粒沉积在MIL-125(Ti)上制备复合光催化剂Ag3PO4/MIL-125(Ti),研究其光催化还原Cr(VI)的性能. ...
Marigold-flower-like TiO2/MIL-125 core-shell composite for enhanced photocatalytic Cr(VI) reduction
1
2021
... 金属有机骨架结构(Metal organic frameworks, MOFs)材料,由金属簇或无机金属离子通过配位键连接而成.MOFs材料具有较大的比表面积、多种金属中心和易于调整的形貌[7,8],可用于光催化领域[9~11].MIL-125(Ti)是一种制备成本低、易于获取的经典MOFs材料,但是较宽的带隙能限制其在光催化领域的应用[12~14].制备复合材料和染料敏化等方式,可提高其光能利用率.Li等[15]用溶剂热法制备了一种以TiO2为壳、以MIL-125(Ti)为芯的核壳结构复合材料,具有良好的光催化还原性能.Hu等[16]制备了MIL-125(Ti)/ZnIn2S4复合材料,Ti4+与Ti3+之间的界面电荷转移和协同作用可高效去除RhB和Cr(VI).Han等[17]通过RhB敏化MIL-125(Ti),光照60 min对甲基橙的降解效率达到90%.Ag3PO4是一种窄带隙(2.45 eV)半导体材料,在可见光区具有光催化活性.Ag3PO4的价带能级较低和导带中有π*轨道,这种特殊的能带结构使其氧化能力和电子迁移速率提高,具有高效降解水体中污染物的性能[18].但是,在光催化过程中Ag3PO4易被光腐蚀,使其光催化效率和循环利用率降低[19,20].将MOFs与Ag3PO4结合制备的复合材料,如Ag/Ag3PO4/HKUST-1[21]、Ag3PO4/MIL-101/NiFe2O4[22]、Ag3PO4/超薄MOF纳米片[23]等,具有优异的光催化性能和稳定性.鉴于此,本文用原位沉积将Ag3PO4纳米颗粒沉积在MIL-125(Ti)上制备复合光催化剂Ag3PO4/MIL-125(Ti),研究其光催化还原Cr(VI)的性能. ...
Construction of MIL-125(Ti)/ZnIn2S4 composites with accelerated interfacial charge transfer for boosting visible light photoreactivity
1
2020
... 金属有机骨架结构(Metal organic frameworks, MOFs)材料,由金属簇或无机金属离子通过配位键连接而成.MOFs材料具有较大的比表面积、多种金属中心和易于调整的形貌[7,8],可用于光催化领域[9~11].MIL-125(Ti)是一种制备成本低、易于获取的经典MOFs材料,但是较宽的带隙能限制其在光催化领域的应用[12~14].制备复合材料和染料敏化等方式,可提高其光能利用率.Li等[15]用溶剂热法制备了一种以TiO2为壳、以MIL-125(Ti)为芯的核壳结构复合材料,具有良好的光催化还原性能.Hu等[16]制备了MIL-125(Ti)/ZnIn2S4复合材料,Ti4+与Ti3+之间的界面电荷转移和协同作用可高效去除RhB和Cr(VI).Han等[17]通过RhB敏化MIL-125(Ti),光照60 min对甲基橙的降解效率达到90%.Ag3PO4是一种窄带隙(2.45 eV)半导体材料,在可见光区具有光催化活性.Ag3PO4的价带能级较低和导带中有π*轨道,这种特殊的能带结构使其氧化能力和电子迁移速率提高,具有高效降解水体中污染物的性能[18].但是,在光催化过程中Ag3PO4易被光腐蚀,使其光催化效率和循环利用率降低[19,20].将MOFs与Ag3PO4结合制备的复合材料,如Ag/Ag3PO4/HKUST-1[21]、Ag3PO4/MIL-101/NiFe2O4[22]、Ag3PO4/超薄MOF纳米片[23]等,具有优异的光催化性能和稳定性.鉴于此,本文用原位沉积将Ag3PO4纳米颗粒沉积在MIL-125(Ti)上制备复合光催化剂Ag3PO4/MIL-125(Ti),研究其光催化还原Cr(VI)的性能. ...
MoS2 quantum dots decorated NH2-MIL-125 heterojunction: preparation and visible light photocatalytic performance
1
2019
... 金属有机骨架结构(Metal organic frameworks, MOFs)材料,由金属簇或无机金属离子通过配位键连接而成.MOFs材料具有较大的比表面积、多种金属中心和易于调整的形貌[7,8],可用于光催化领域[9~11].MIL-125(Ti)是一种制备成本低、易于获取的经典MOFs材料,但是较宽的带隙能限制其在光催化领域的应用[12~14].制备复合材料和染料敏化等方式,可提高其光能利用率.Li等[15]用溶剂热法制备了一种以TiO2为壳、以MIL-125(Ti)为芯的核壳结构复合材料,具有良好的光催化还原性能.Hu等[16]制备了MIL-125(Ti)/ZnIn2S4复合材料,Ti4+与Ti3+之间的界面电荷转移和协同作用可高效去除RhB和Cr(VI).Han等[17]通过RhB敏化MIL-125(Ti),光照60 min对甲基橙的降解效率达到90%.Ag3PO4是一种窄带隙(2.45 eV)半导体材料,在可见光区具有光催化活性.Ag3PO4的价带能级较低和导带中有π*轨道,这种特殊的能带结构使其氧化能力和电子迁移速率提高,具有高效降解水体中污染物的性能[18].但是,在光催化过程中Ag3PO4易被光腐蚀,使其光催化效率和循环利用率降低[19,20].将MOFs与Ag3PO4结合制备的复合材料,如Ag/Ag3PO4/HKUST-1[21]、Ag3PO4/MIL-101/NiFe2O4[22]、Ag3PO4/超薄MOF纳米片[23]等,具有优异的光催化性能和稳定性.鉴于此,本文用原位沉积将Ag3PO4纳米颗粒沉积在MIL-125(Ti)上制备复合光催化剂Ag3PO4/MIL-125(Ti),研究其光催化还原Cr(VI)的性能. ...
具有可见光催化活性的MoS2量子点/NH2-MIL-125复合材料的制备及性能表征
1
2019
... 金属有机骨架结构(Metal organic frameworks, MOFs)材料,由金属簇或无机金属离子通过配位键连接而成.MOFs材料具有较大的比表面积、多种金属中心和易于调整的形貌[7,8],可用于光催化领域[9~11].MIL-125(Ti)是一种制备成本低、易于获取的经典MOFs材料,但是较宽的带隙能限制其在光催化领域的应用[12~14].制备复合材料和染料敏化等方式,可提高其光能利用率.Li等[15]用溶剂热法制备了一种以TiO2为壳、以MIL-125(Ti)为芯的核壳结构复合材料,具有良好的光催化还原性能.Hu等[16]制备了MIL-125(Ti)/ZnIn2S4复合材料,Ti4+与Ti3+之间的界面电荷转移和协同作用可高效去除RhB和Cr(VI).Han等[17]通过RhB敏化MIL-125(Ti),光照60 min对甲基橙的降解效率达到90%.Ag3PO4是一种窄带隙(2.45 eV)半导体材料,在可见光区具有光催化活性.Ag3PO4的价带能级较低和导带中有π*轨道,这种特殊的能带结构使其氧化能力和电子迁移速率提高,具有高效降解水体中污染物的性能[18].但是,在光催化过程中Ag3PO4易被光腐蚀,使其光催化效率和循环利用率降低[19,20].将MOFs与Ag3PO4结合制备的复合材料,如Ag/Ag3PO4/HKUST-1[21]、Ag3PO4/MIL-101/NiFe2O4[22]、Ag3PO4/超薄MOF纳米片[23]等,具有优异的光催化性能和稳定性.鉴于此,本文用原位沉积将Ag3PO4纳米颗粒沉积在MIL-125(Ti)上制备复合光催化剂Ag3PO4/MIL-125(Ti),研究其光催化还原Cr(VI)的性能. ...
One-step microwave-hydrothermal synthesis of visible-light-driven Ag3PO4/LaPO4 photocatalyst induced by visible light irradiation
1
2021
... 金属有机骨架结构(Metal organic frameworks, MOFs)材料,由金属簇或无机金属离子通过配位键连接而成.MOFs材料具有较大的比表面积、多种金属中心和易于调整的形貌[7,8],可用于光催化领域[9~11].MIL-125(Ti)是一种制备成本低、易于获取的经典MOFs材料,但是较宽的带隙能限制其在光催化领域的应用[12~14].制备复合材料和染料敏化等方式,可提高其光能利用率.Li等[15]用溶剂热法制备了一种以TiO2为壳、以MIL-125(Ti)为芯的核壳结构复合材料,具有良好的光催化还原性能.Hu等[16]制备了MIL-125(Ti)/ZnIn2S4复合材料,Ti4+与Ti3+之间的界面电荷转移和协同作用可高效去除RhB和Cr(VI).Han等[17]通过RhB敏化MIL-125(Ti),光照60 min对甲基橙的降解效率达到90%.Ag3PO4是一种窄带隙(2.45 eV)半导体材料,在可见光区具有光催化活性.Ag3PO4的价带能级较低和导带中有π*轨道,这种特殊的能带结构使其氧化能力和电子迁移速率提高,具有高效降解水体中污染物的性能[18].但是,在光催化过程中Ag3PO4易被光腐蚀,使其光催化效率和循环利用率降低[19,20].将MOFs与Ag3PO4结合制备的复合材料,如Ag/Ag3PO4/HKUST-1[21]、Ag3PO4/MIL-101/NiFe2O4[22]、Ag3PO4/超薄MOF纳米片[23]等,具有优异的光催化性能和稳定性.鉴于此,本文用原位沉积将Ag3PO4纳米颗粒沉积在MIL-125(Ti)上制备复合光催化剂Ag3PO4/MIL-125(Ti),研究其光催化还原Cr(VI)的性能. ...
Photo-assisted synthesis of Ag3PO4/reduced graphene oxide/Ag heterostructure photocatalyst with enhanced photocatalytic activity and stability under visible light
1
2014
... 金属有机骨架结构(Metal organic frameworks, MOFs)材料,由金属簇或无机金属离子通过配位键连接而成.MOFs材料具有较大的比表面积、多种金属中心和易于调整的形貌[7,8],可用于光催化领域[9~11].MIL-125(Ti)是一种制备成本低、易于获取的经典MOFs材料,但是较宽的带隙能限制其在光催化领域的应用[12~14].制备复合材料和染料敏化等方式,可提高其光能利用率.Li等[15]用溶剂热法制备了一种以TiO2为壳、以MIL-125(Ti)为芯的核壳结构复合材料,具有良好的光催化还原性能.Hu等[16]制备了MIL-125(Ti)/ZnIn2S4复合材料,Ti4+与Ti3+之间的界面电荷转移和协同作用可高效去除RhB和Cr(VI).Han等[17]通过RhB敏化MIL-125(Ti),光照60 min对甲基橙的降解效率达到90%.Ag3PO4是一种窄带隙(2.45 eV)半导体材料,在可见光区具有光催化活性.Ag3PO4的价带能级较低和导带中有π*轨道,这种特殊的能带结构使其氧化能力和电子迁移速率提高,具有高效降解水体中污染物的性能[18].但是,在光催化过程中Ag3PO4易被光腐蚀,使其光催化效率和循环利用率降低[19,20].将MOFs与Ag3PO4结合制备的复合材料,如Ag/Ag3PO4/HKUST-1[21]、Ag3PO4/MIL-101/NiFe2O4[22]、Ag3PO4/超薄MOF纳米片[23]等,具有优异的光催化性能和稳定性.鉴于此,本文用原位沉积将Ag3PO4纳米颗粒沉积在MIL-125(Ti)上制备复合光催化剂Ag3PO4/MIL-125(Ti),研究其光催化还原Cr(VI)的性能. ...
The stabilization effect of surface capping on photocatalytic activity and recyclable stability of Ag3PO4
1
2014
... 金属有机骨架结构(Metal organic frameworks, MOFs)材料,由金属簇或无机金属离子通过配位键连接而成.MOFs材料具有较大的比表面积、多种金属中心和易于调整的形貌[7,8],可用于光催化领域[9~11].MIL-125(Ti)是一种制备成本低、易于获取的经典MOFs材料,但是较宽的带隙能限制其在光催化领域的应用[12~14].制备复合材料和染料敏化等方式,可提高其光能利用率.Li等[15]用溶剂热法制备了一种以TiO2为壳、以MIL-125(Ti)为芯的核壳结构复合材料,具有良好的光催化还原性能.Hu等[16]制备了MIL-125(Ti)/ZnIn2S4复合材料,Ti4+与Ti3+之间的界面电荷转移和协同作用可高效去除RhB和Cr(VI).Han等[17]通过RhB敏化MIL-125(Ti),光照60 min对甲基橙的降解效率达到90%.Ag3PO4是一种窄带隙(2.45 eV)半导体材料,在可见光区具有光催化活性.Ag3PO4的价带能级较低和导带中有π*轨道,这种特殊的能带结构使其氧化能力和电子迁移速率提高,具有高效降解水体中污染物的性能[18].但是,在光催化过程中Ag3PO4易被光腐蚀,使其光催化效率和循环利用率降低[19,20].将MOFs与Ag3PO4结合制备的复合材料,如Ag/Ag3PO4/HKUST-1[21]、Ag3PO4/MIL-101/NiFe2O4[22]、Ag3PO4/超薄MOF纳米片[23]等,具有优异的光催化性能和稳定性.鉴于此,本文用原位沉积将Ag3PO4纳米颗粒沉积在MIL-125(Ti)上制备复合光催化剂Ag3PO4/MIL-125(Ti),研究其光催化还原Cr(VI)的性能. ...
Enhancement of the photocatalytic performance and thermal stability of an iron based metal–organic-framework functionalised by Ag/Ag3PO4
1
2018
... 金属有机骨架结构(Metal organic frameworks, MOFs)材料,由金属簇或无机金属离子通过配位键连接而成.MOFs材料具有较大的比表面积、多种金属中心和易于调整的形貌[7,8],可用于光催化领域[9~11].MIL-125(Ti)是一种制备成本低、易于获取的经典MOFs材料,但是较宽的带隙能限制其在光催化领域的应用[12~14].制备复合材料和染料敏化等方式,可提高其光能利用率.Li等[15]用溶剂热法制备了一种以TiO2为壳、以MIL-125(Ti)为芯的核壳结构复合材料,具有良好的光催化还原性能.Hu等[16]制备了MIL-125(Ti)/ZnIn2S4复合材料,Ti4+与Ti3+之间的界面电荷转移和协同作用可高效去除RhB和Cr(VI).Han等[17]通过RhB敏化MIL-125(Ti),光照60 min对甲基橙的降解效率达到90%.Ag3PO4是一种窄带隙(2.45 eV)半导体材料,在可见光区具有光催化活性.Ag3PO4的价带能级较低和导带中有π*轨道,这种特殊的能带结构使其氧化能力和电子迁移速率提高,具有高效降解水体中污染物的性能[18].但是,在光催化过程中Ag3PO4易被光腐蚀,使其光催化效率和循环利用率降低[19,20].将MOFs与Ag3PO4结合制备的复合材料,如Ag/Ag3PO4/HKUST-1[21]、Ag3PO4/MIL-101/NiFe2O4[22]、Ag3PO4/超薄MOF纳米片[23]等,具有优异的光催化性能和稳定性.鉴于此,本文用原位沉积将Ag3PO4纳米颗粒沉积在MIL-125(Ti)上制备复合光催化剂Ag3PO4/MIL-125(Ti),研究其光催化还原Cr(VI)的性能. ...
Highly efficient visible-light-driven photocatalytic degradation of rhodamine B by a novel Z-scheme Ag3PO4/MIL-101/NiFe2O4 composite
1
2018
... 金属有机骨架结构(Metal organic frameworks, MOFs)材料,由金属簇或无机金属离子通过配位键连接而成.MOFs材料具有较大的比表面积、多种金属中心和易于调整的形貌[7,8],可用于光催化领域[9~11].MIL-125(Ti)是一种制备成本低、易于获取的经典MOFs材料,但是较宽的带隙能限制其在光催化领域的应用[12~14].制备复合材料和染料敏化等方式,可提高其光能利用率.Li等[15]用溶剂热法制备了一种以TiO2为壳、以MIL-125(Ti)为芯的核壳结构复合材料,具有良好的光催化还原性能.Hu等[16]制备了MIL-125(Ti)/ZnIn2S4复合材料,Ti4+与Ti3+之间的界面电荷转移和协同作用可高效去除RhB和Cr(VI).Han等[17]通过RhB敏化MIL-125(Ti),光照60 min对甲基橙的降解效率达到90%.Ag3PO4是一种窄带隙(2.45 eV)半导体材料,在可见光区具有光催化活性.Ag3PO4的价带能级较低和导带中有π*轨道,这种特殊的能带结构使其氧化能力和电子迁移速率提高,具有高效降解水体中污染物的性能[18].但是,在光催化过程中Ag3PO4易被光腐蚀,使其光催化效率和循环利用率降低[19,20].将MOFs与Ag3PO4结合制备的复合材料,如Ag/Ag3PO4/HKUST-1[21]、Ag3PO4/MIL-101/NiFe2O4[22]、Ag3PO4/超薄MOF纳米片[23]等,具有优异的光催化性能和稳定性.鉴于此,本文用原位沉积将Ag3PO4纳米颗粒沉积在MIL-125(Ti)上制备复合光催化剂Ag3PO4/MIL-125(Ti),研究其光催化还原Cr(VI)的性能. ...
Ag3PO4@UMOFNs core–shell structure: two-dimensional MOFs promoted photoinduced charge separation and Photocatalysis
1
2018
... 金属有机骨架结构(Metal organic frameworks, MOFs)材料,由金属簇或无机金属离子通过配位键连接而成.MOFs材料具有较大的比表面积、多种金属中心和易于调整的形貌[7,8],可用于光催化领域[9~11].MIL-125(Ti)是一种制备成本低、易于获取的经典MOFs材料,但是较宽的带隙能限制其在光催化领域的应用[12~14].制备复合材料和染料敏化等方式,可提高其光能利用率.Li等[15]用溶剂热法制备了一种以TiO2为壳、以MIL-125(Ti)为芯的核壳结构复合材料,具有良好的光催化还原性能.Hu等[16]制备了MIL-125(Ti)/ZnIn2S4复合材料,Ti4+与Ti3+之间的界面电荷转移和协同作用可高效去除RhB和Cr(VI).Han等[17]通过RhB敏化MIL-125(Ti),光照60 min对甲基橙的降解效率达到90%.Ag3PO4是一种窄带隙(2.45 eV)半导体材料,在可见光区具有光催化活性.Ag3PO4的价带能级较低和导带中有π*轨道,这种特殊的能带结构使其氧化能力和电子迁移速率提高,具有高效降解水体中污染物的性能[18].但是,在光催化过程中Ag3PO4易被光腐蚀,使其光催化效率和循环利用率降低[19,20].将MOFs与Ag3PO4结合制备的复合材料,如Ag/Ag3PO4/HKUST-1[21]、Ag3PO4/MIL-101/NiFe2O4[22]、Ag3PO4/超薄MOF纳米片[23]等,具有优异的光催化性能和稳定性.鉴于此,本文用原位沉积将Ag3PO4纳米颗粒沉积在MIL-125(Ti)上制备复合光催化剂Ag3PO4/MIL-125(Ti),研究其光催化还原Cr(VI)的性能. ...
Boosting visible light photocatalytic activity via impregnation-induced RhB-sensitized MIL-125(Ti)
1
2019
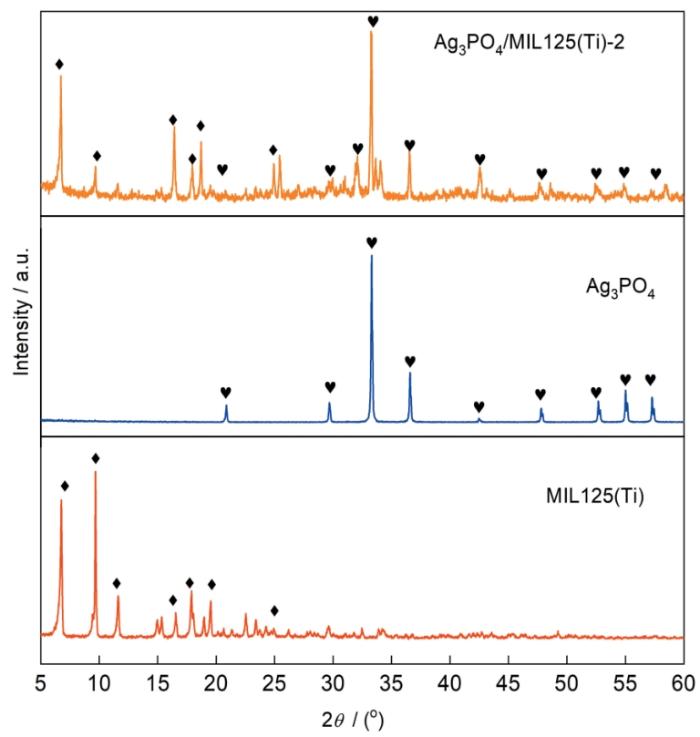
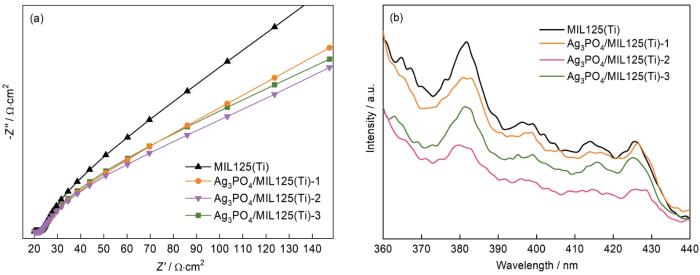
... 图1给出了不同样品的XRD谱.可以看出,在MIL125(Ti)的谱中衍射峰分别出现在6.79°、9.83°、11.69°、15.06°、15.45°、16.63°、17.92°、19.07°和19.64°处,与文献中MIL125(Ti)衍射峰的位置一致[24].在Ag3PO4的谱中位于20.9°、29.7°、33.3°、36.6°、47.8°、52.7°、55.0°和57.3°的特征衍射峰分别对应(110),(200),(210),(211),(310),(222),(320)和(321)晶面[25].在Ag3PO4/MIL125(Ti)-2的谱中可观察到MIL125(Ti)和Ag3PO4的两组特征衍射峰,表明用原位沉积法可制备Ag3PO4/MIL125(Ti)复合光催化剂. ...
A stable Ag3PO4@PANI core@shell hybrid: Enrichment photocatalytic degradation with π-π conjugation
1
2017
... 图1给出了不同样品的XRD谱.可以看出,在MIL125(Ti)的谱中衍射峰分别出现在6.79°、9.83°、11.69°、15.06°、15.45°、16.63°、17.92°、19.07°和19.64°处,与文献中MIL125(Ti)衍射峰的位置一致[24].在Ag3PO4的谱中位于20.9°、29.7°、33.3°、36.6°、47.8°、52.7°、55.0°和57.3°的特征衍射峰分别对应(110),(200),(210),(211),(310),(222),(320)和(321)晶面[25].在Ag3PO4/MIL125(Ti)-2的谱中可观察到MIL125(Ti)和Ag3PO4的两组特征衍射峰,表明用原位沉积法可制备Ag3PO4/MIL125(Ti)复合光催化剂. ...
Construction of MIL-53(Fe) metal-organic framework modified by silver phosphate nanoparticles as a novel Z-scheme photocatalyst: Visible-light photocatalytic performance and mechanism investigation
1
2019
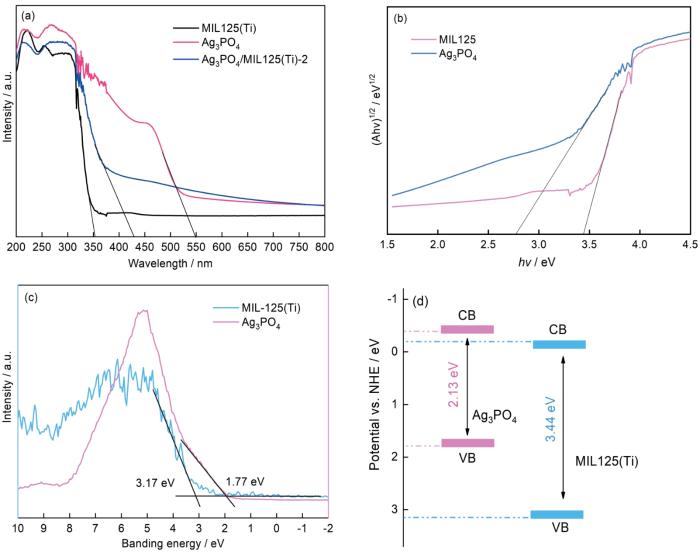
... 从图3a可见,MIL125(Ti)的吸收光谱集中在紫外光区,吸收边界为354 nm.Ag3PO4在紫外光谱和可见光区都有较强的吸收能力,其吸收边界为550 nm.与MIL-125(Ti)相比,Ag3PO4/MIL125(Ti)-2的光吸收范围扩展到可见光区,吸收边界为442 nm.这表明,MIL125(Ti)与Ag3PO4复合能有效提高光吸收能力,使Ag3PO4/MIL125(Ti)-2能被可见光激发进行光催化反应[26].图3b表明,MIL125(Ti)和Ag3PO4带隙能分别为3.44 eV和2.13 eV.图3c表明,由价带谱测得的MIL-125(Ti)的HUMO能级和Ag3PO4的价带(VB)电位分别为3.17 eV和1.77 eV.根据公式[27] ...
Photocatalytic degradation of organic contaminants by magnetic Ag3PO4/MFe2O4 (M = Zn, Ni, Co) composites: a comparative study and a new insight into mechanism
1
2021
... 从图3a可见,MIL125(Ti)的吸收光谱集中在紫外光区,吸收边界为354 nm.Ag3PO4在紫外光谱和可见光区都有较强的吸收能力,其吸收边界为550 nm.与MIL-125(Ti)相比,Ag3PO4/MIL125(Ti)-2的光吸收范围扩展到可见光区,吸收边界为442 nm.这表明,MIL125(Ti)与Ag3PO4复合能有效提高光吸收能力,使Ag3PO4/MIL125(Ti)-2能被可见光激发进行光催化反应[26].图3b表明,MIL125(Ti)和Ag3PO4带隙能分别为3.44 eV和2.13 eV.图3c表明,由价带谱测得的MIL-125(Ti)的HUMO能级和Ag3PO4的价带(VB)电位分别为3.17 eV和1.77 eV.根据公式[27] ...
Interface electron transfer of Bi2MoO6/MIL-125 and the visible-light performance for pollutant degradation
1
2020
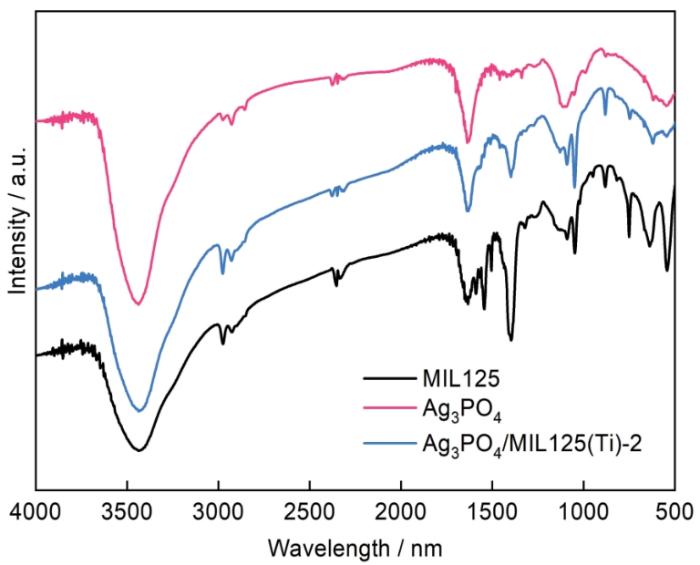
... 图4给出了用FTIR测定的不同样品的价键结构.所有样品在3430 cm-1处的宽吸收峰归因于吸附在样品表面的残余水分子H—O—H的弯曲振动[28].在MIL125(Ti)的红外光谱中,位于400~800 cm-1范围内的吸收峰是Ti—O—Ti振动引起[29],位于1410、1597和1700 cm-1处的吸收峰则分别对应O—C—O的对称伸缩振动[29]、苯环的C=C振动[30]和芳香羧酸[31].在Ag3PO4/MIL125(Ti)-2的红外光谱中,除属于MIL125(Ti)的吸收峰以外,位于537和989 cm-1处的两个吸收峰与磷酸盐中P—O的伸缩振动有关[32].对红外光谱分析进一步证明,已经成功地制备出Ag3PO4和MIL125(Ti)复合光催化剂. ...
Enhanced photocatalytic activity of MIL-125 by post-synthetic modification with CrIII and Ag nanoparticles
2
2015
... 图4给出了用FTIR测定的不同样品的价键结构.所有样品在3430 cm-1处的宽吸收峰归因于吸附在样品表面的残余水分子H—O—H的弯曲振动[28].在MIL125(Ti)的红外光谱中,位于400~800 cm-1范围内的吸收峰是Ti—O—Ti振动引起[29],位于1410、1597和1700 cm-1处的吸收峰则分别对应O—C—O的对称伸缩振动[29]、苯环的C=C振动[30]和芳香羧酸[31].在Ag3PO4/MIL125(Ti)-2的红外光谱中,除属于MIL125(Ti)的吸收峰以外,位于537和989 cm-1处的两个吸收峰与磷酸盐中P—O的伸缩振动有关[32].对红外光谱分析进一步证明,已经成功地制备出Ag3PO4和MIL125(Ti)复合光催化剂. ...
... [29]、苯环的C=C振动[30]和芳香羧酸[31].在Ag3PO4/MIL125(Ti)-2的红外光谱中,除属于MIL125(Ti)的吸收峰以外,位于537和989 cm-1处的两个吸收峰与磷酸盐中P—O的伸缩振动有关[32].对红外光谱分析进一步证明,已经成功地制备出Ag3PO4和MIL125(Ti)复合光催化剂. ...
NH2-MIL-125(Ti) and its emeraldine functionalized derivative as a chemical sensor for effective detection of dopamine
1
2019
... 图4给出了用FTIR测定的不同样品的价键结构.所有样品在3430 cm-1处的宽吸收峰归因于吸附在样品表面的残余水分子H—O—H的弯曲振动[28].在MIL125(Ti)的红外光谱中,位于400~800 cm-1范围内的吸收峰是Ti—O—Ti振动引起[29],位于1410、1597和1700 cm-1处的吸收峰则分别对应O—C—O的对称伸缩振动[29]、苯环的C=C振动[30]和芳香羧酸[31].在Ag3PO4/MIL125(Ti)-2的红外光谱中,除属于MIL125(Ti)的吸收峰以外,位于537和989 cm-1处的两个吸收峰与磷酸盐中P—O的伸缩振动有关[32].对红外光谱分析进一步证明,已经成功地制备出Ag3PO4和MIL125(Ti)复合光催化剂. ...
Enhanced photocatalytic performance of BiOBr/NH2-MIL-125(Ti) composite for dye degradation under visible light
1
2016
... 图4给出了用FTIR测定的不同样品的价键结构.所有样品在3430 cm-1处的宽吸收峰归因于吸附在样品表面的残余水分子H—O—H的弯曲振动[28].在MIL125(Ti)的红外光谱中,位于400~800 cm-1范围内的吸收峰是Ti—O—Ti振动引起[29],位于1410、1597和1700 cm-1处的吸收峰则分别对应O—C—O的对称伸缩振动[29]、苯环的C=C振动[30]和芳香羧酸[31].在Ag3PO4/MIL125(Ti)-2的红外光谱中,除属于MIL125(Ti)的吸收峰以外,位于537和989 cm-1处的两个吸收峰与磷酸盐中P—O的伸缩振动有关[32].对红外光谱分析进一步证明,已经成功地制备出Ag3PO4和MIL125(Ti)复合光催化剂. ...
In-situ synthesis of facet-dependent BiVO4/Ag3PO4/PANI photocatalyst with enhanced visible-light-induced photocatalytic degradation performance: synergism of interfacial coupling and hole-transfer
1
2020
... 图4给出了用FTIR测定的不同样品的价键结构.所有样品在3430 cm-1处的宽吸收峰归因于吸附在样品表面的残余水分子H—O—H的弯曲振动[28].在MIL125(Ti)的红外光谱中,位于400~800 cm-1范围内的吸收峰是Ti—O—Ti振动引起[29],位于1410、1597和1700 cm-1处的吸收峰则分别对应O—C—O的对称伸缩振动[29]、苯环的C=C振动[30]和芳香羧酸[31].在Ag3PO4/MIL125(Ti)-2的红外光谱中,除属于MIL125(Ti)的吸收峰以外,位于537和989 cm-1处的两个吸收峰与磷酸盐中P—O的伸缩振动有关[32].对红外光谱分析进一步证明,已经成功地制备出Ag3PO4和MIL125(Ti)复合光催化剂. ...
Effective degradation of norfloxacin on Ag3PO4/CNTs photoanode: Z-scheme mechanism, reaction pathway, and toxicity assessment
1
2022
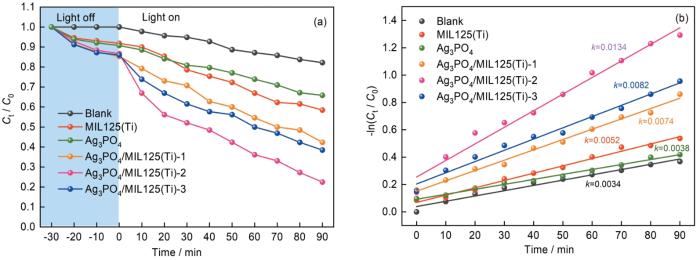
... 以容积为100 mL、浓度为10 mg/L的Cr(VI)溶液作为底物,考察了样品在模拟太阳光下的还原性能.图6a表明,暗反应30 min后光催化体系达到吸附-解吸平衡.不加光催化剂光照90 min后Cr(VI)还原率为17.8%,使用MIL125(Ti)和Ag3PO4光催化剂对Cr(VI)的还原率分别为41.6%和34.2%.这表明,二者光催化还原Cr(VI)的性能有限.MIL125(Ti)和Ag3PO4二者复合对Cr(VI)的光催化还原率明显提高,Ag3PO4/MIL125(Ti)-2的光催化还原性能最佳.光照90 min对Cr(VI)的还原率为77.6%,还原速率k为0.0132 min-1,分别是MIL125(Ti)和Ag3PO4的2.58倍和3.53倍.其原因是,Ag3PO4的引入拓展了复合光催化剂的吸光范围,并促进了光生载流子的分离[33].值得注意的是,复合光催化剂中Ag3PO4的质量比升至30%后Ag3PO4/MIL125(Ti)-3对Cr(VI)的还原率反而降低至41.6%,可能是过量的Ag3PO4纳米颗粒团聚使光生载流子重新复合所致[34]. ...
One-pot synthesis of CeO2/Mg-Al layered double oxide nanosheets for efficient visible-light induced photo-reduction of Cr(VI)
2
2020
... 以容积为100 mL、浓度为10 mg/L的Cr(VI)溶液作为底物,考察了样品在模拟太阳光下的还原性能.图6a表明,暗反应30 min后光催化体系达到吸附-解吸平衡.不加光催化剂光照90 min后Cr(VI)还原率为17.8%,使用MIL125(Ti)和Ag3PO4光催化剂对Cr(VI)的还原率分别为41.6%和34.2%.这表明,二者光催化还原Cr(VI)的性能有限.MIL125(Ti)和Ag3PO4二者复合对Cr(VI)的光催化还原率明显提高,Ag3PO4/MIL125(Ti)-2的光催化还原性能最佳.光照90 min对Cr(VI)的还原率为77.6%,还原速率k为0.0132 min-1,分别是MIL125(Ti)和Ag3PO4的2.58倍和3.53倍.其原因是,Ag3PO4的引入拓展了复合光催化剂的吸光范围,并促进了光生载流子的分离[33].值得注意的是,复合光催化剂中Ag3PO4的质量比升至30%后Ag3PO4/MIL125(Ti)-3对Cr(VI)的还原率反而降低至41.6%,可能是过量的Ag3PO4纳米颗粒团聚使光生载流子重新复合所致[34]. ...
... 图7c、d给出了光催化剂投加量对其还原Cr(VI)性能的影响.可以看出,随着Ag3PO4/MIL-125(Ti)-2投加量的增大Cr(VI)的光催化还原率呈先提高后降低的趋势.Ag3PO4/MIL-125(Ti)-2的投加量为30 mg时Cr(VI)的光催化还原率最高,达到94.9%.其原因是,随着催化剂质量的增加光生e-/h+对的数量随之增加[38].当Ag3PO4/MIL-125(Ti)-2的投加量升至50 mg时,对Cr(VI)的光催化还原率反而下降至84.7%.其原因是,过量的光催化剂分散在溶液中,颗粒的散射导致对光的利用率降低[34]. ...
Insight into highly efficient simultaneous photocatalytic removal of Cr(VI) and 2,4-diclorophenol under visible light irradiation by phosphorus doped porous ultrathin g-C3N4 nanosheets from aqueous media: Performance and reaction mechanism
1
2017
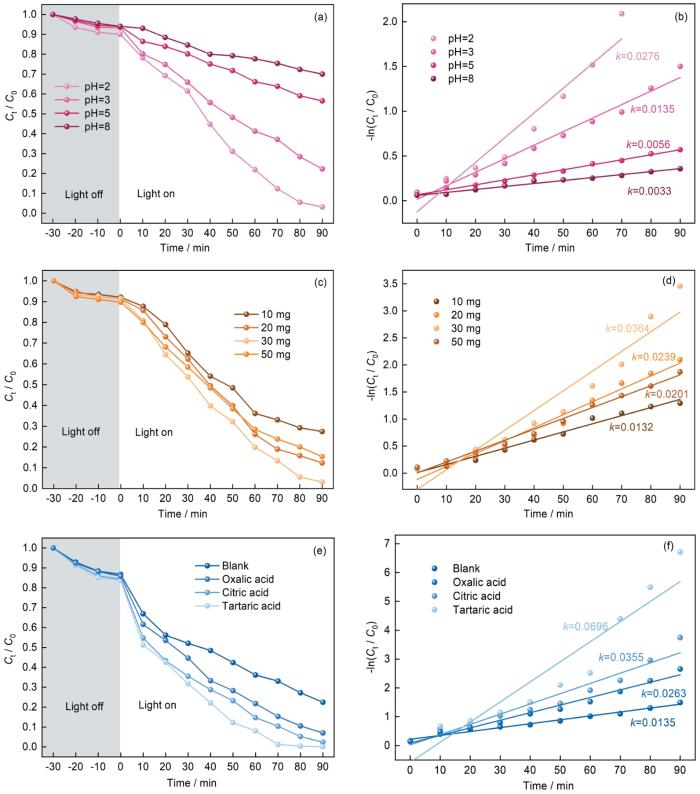
... 溶液的pH值对Cr(VI)的还原效率有较大的影响 [35],因此以Ag3PO4/MIL125(Ti)-2为光催化剂,用H2SO4和NaOH调节Cr(VI)溶液的初始pH值,考察了Ag3PO4/MIL125(Ti)-2对Cr(VI)的光催化还原性能.从图7a、b可见,在酸性条件下Ag3PO4/MIL-125(Ti)-2对Cr(VI)的光催化还原速率明显高于碱性和中性条件.溶液的初始pH值为2时Ag3PO4/MIL-125(Ti)-2的光催化还原性能最佳.模拟太阳光光照90 min后Cr(VI)的还原率为96.9%,其反应速率常数k为0.0276 min-1.其原因是,酸性条件有助于Cr2O7-和HCrO4-与H+快速反应生成Cr3+(式(2)和(3))[36].同时,pH值不同产物Cr3+的存在形式也不同(式(2)~(4)).在中性和碱性条件下生成的Cr(OH)3容易沉积在MIL125(Ti)孔径中,使活性位点减少和反应速率降低[37]. ...
Reduced graphene oxide enwrapped pinecone-liked Ag3PO4/TiO2 composites with enhanced photocatalytic activity and stability under visible light
1
2015
... 溶液的pH值对Cr(VI)的还原效率有较大的影响 [35],因此以Ag3PO4/MIL125(Ti)-2为光催化剂,用H2SO4和NaOH调节Cr(VI)溶液的初始pH值,考察了Ag3PO4/MIL125(Ti)-2对Cr(VI)的光催化还原性能.从图7a、b可见,在酸性条件下Ag3PO4/MIL-125(Ti)-2对Cr(VI)的光催化还原速率明显高于碱性和中性条件.溶液的初始pH值为2时Ag3PO4/MIL-125(Ti)-2的光催化还原性能最佳.模拟太阳光光照90 min后Cr(VI)的还原率为96.9%,其反应速率常数k为0.0276 min-1.其原因是,酸性条件有助于Cr2O7-和HCrO4-与H+快速反应生成Cr3+(式(2)和(3))[36].同时,pH值不同产物Cr3+的存在形式也不同(式(2)~(4)).在中性和碱性条件下生成的Cr(OH)3容易沉积在MIL125(Ti)孔径中,使活性位点减少和反应速率降低[37]. ...
One-pot fabrication of β-Bi2O3@Bi2S3 hierarchical hollow spheres with advanced sunlight photocatalytic RhB oxidation and Cr(VI) reduction activities
1
2018
... 溶液的pH值对Cr(VI)的还原效率有较大的影响 [35],因此以Ag3PO4/MIL125(Ti)-2为光催化剂,用H2SO4和NaOH调节Cr(VI)溶液的初始pH值,考察了Ag3PO4/MIL125(Ti)-2对Cr(VI)的光催化还原性能.从图7a、b可见,在酸性条件下Ag3PO4/MIL-125(Ti)-2对Cr(VI)的光催化还原速率明显高于碱性和中性条件.溶液的初始pH值为2时Ag3PO4/MIL-125(Ti)-2的光催化还原性能最佳.模拟太阳光光照90 min后Cr(VI)的还原率为96.9%,其反应速率常数k为0.0276 min-1.其原因是,酸性条件有助于Cr2O7-和HCrO4-与H+快速反应生成Cr3+(式(2)和(3))[36].同时,pH值不同产物Cr3+的存在形式也不同(式(2)~(4)).在中性和碱性条件下生成的Cr(OH)3容易沉积在MIL125(Ti)孔径中,使活性位点减少和反应速率降低[37]. ...
Facile synthesis a novel core-shell amino functionalized MIL-125(Ti) micro-photocatalyst for enhanced degradation of tetracycline hydrochloride under visible light
1
2021
... 图7c、d给出了光催化剂投加量对其还原Cr(VI)性能的影响.可以看出,随着Ag3PO4/MIL-125(Ti)-2投加量的增大Cr(VI)的光催化还原率呈先提高后降低的趋势.Ag3PO4/MIL-125(Ti)-2的投加量为30 mg时Cr(VI)的光催化还原率最高,达到94.9%.其原因是,随着催化剂质量的增加光生e-/h+对的数量随之增加[38].当Ag3PO4/MIL-125(Ti)-2的投加量升至50 mg时,对Cr(VI)的光催化还原率反而下降至84.7%.其原因是,过量的光催化剂分散在溶液中,颗粒的散射导致对光的利用率降低[34]. ...
Facile fabrication of BUC-21/g-C3N4 composites and their enhanced photocatalytic Cr(VI) reduction performances under simulated sunlight
1
2019
... 以柠檬树、酒石酸和草酸为小分子有机酸,对Cr(VI)光催化还原效率的影响如图7e、f所示.加入小分子有机酸不同程度地促进了Ag3PO4/MIL-125(Ti)-2光催化还原Cr(VI)的效率.加入酒石酸后光催化还原Cr(VI)反应速率常数k达到0.0696 min-1,是不加入小分子有机酸时(k=0.0135 min-1)的5.16倍.其原因是,小分子有机酸与光生h+发生反应促进了光生e-和h+的分离,使参与还原Cr(VI)的光生e-增多[39]. ...
Metal nanoparticles decorated MIL-125-NH2 and MIL-125 for efficient photocatalysis
1
2019
... / mg·L
-1Irradiation | pH | Efficiency /% | Ref. | | Pt/MIL-125-NH2/1000 | Cr(VI)/15 | 300 W Xe lamp | 6 | 75.0% (120 min) | [40] |
| Bi2S3@NH2-MIL-125(Ti)/100 | Cr(VI)/10 | 300 W Xe lamp | 7 | 77.0% (120 min) | [41] |
| CdS/MIL-125(Ti)/500 | Cr(VI)/48 | 300 W Xe lamp(cut~420 nm) | 6 | 35.0% (70 min) | [42] |
| Pd@MIL-101/1000 | Cr(VI)/10 | 125 W light pressure mercury lamp | 6 | 46.4% (240 min) | [43] |
| MIL-125-derived TiO2@C/300 | Cr(VI)/5 | – | 3 | 61.8% (90 min) | [44] |
| Ag3PO4/MIL-125(Ti)-2 | Cr(VI)/10 | 300 W Xe lamp | 6 | 77.6% (90 min) | This work |
| Ag3PO4/MIL-125(Ti)-2 | Cr(VI)/10 | 300 W Xe lamp | 2 | 96.9% (90 min) | This work |
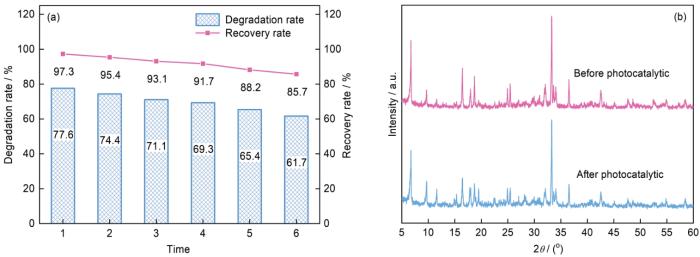
2.4.3 光催化剂的稳定性进行6次光催化循环实验,考察Ag3PO4/MIL-125(Ti)-2的稳定性.图8a表明,4次光催化循环实验后Ag3PO4/MIL-125(Ti)-2的回收率为90.7%,对Cr(VI)的光催化还原率仍保持较高的水平(69.3%).循环次数增至6次后Ag3PO4/MIL-125(Ti)-2对Cr(VI)的光催化还原率由77.6%降低至61.7%,其原因可能是在光催化过程中催化剂损耗.图8b给出了Ag3PO4/MIL-125(Ti)-2在6次光催化反应前后XRD谱的对比.可以看出,经过光催化反应后Ag3PO4/MIL-125(Ti)-2中对应的Ag3PO4和MIL-125(Ti)特征峰位置没有变化,说明二者的晶型没有改变,表明催化剂具有良好的稳定性. ...
Heterostructured Bi2S3@NH2-MIL-125(Ti) nanocomposite as a bifunctional photocatalyst for Cr(vi) reduction and rhodamine B degradation under visible light
1
2018
... / mg·L
-1Irradiation | pH | Efficiency /% | Ref. | | Pt/MIL-125-NH2/1000 | Cr(VI)/15 | 300 W Xe lamp | 6 | 75.0% (120 min) | [40] |
| Bi2S3@NH2-MIL-125(Ti)/100 | Cr(VI)/10 | 300 W Xe lamp | 7 | 77.0% (120 min) | [41] |
| CdS/MIL-125(Ti)/500 | Cr(VI)/48 | 300 W Xe lamp(cut~420 nm) | 6 | 35.0% (70 min) | [42] |
| Pd@MIL-101/1000 | Cr(VI)/10 | 125 W light pressure mercury lamp | 6 | 46.4% (240 min) | [43] |
| MIL-125-derived TiO2@C/300 | Cr(VI)/5 | – | 3 | 61.8% (90 min) | [44] |
| Ag3PO4/MIL-125(Ti)-2 | Cr(VI)/10 | 300 W Xe lamp | 6 | 77.6% (90 min) | This work |
| Ag3PO4/MIL-125(Ti)-2 | Cr(VI)/10 | 300 W Xe lamp | 2 | 96.9% (90 min) | This work |
2.4.3 光催化剂的稳定性进行6次光催化循环实验,考察Ag3PO4/MIL-125(Ti)-2的稳定性.图8a表明,4次光催化循环实验后Ag3PO4/MIL-125(Ti)-2的回收率为90.7%,对Cr(VI)的光催化还原率仍保持较高的水平(69.3%).循环次数增至6次后Ag3PO4/MIL-125(Ti)-2对Cr(VI)的光催化还原率由77.6%降低至61.7%,其原因可能是在光催化过程中催化剂损耗.图8b给出了Ag3PO4/MIL-125(Ti)-2在6次光催化反应前后XRD谱的对比.可以看出,经过光催化反应后Ag3PO4/MIL-125(Ti)-2中对应的Ag3PO4和MIL-125(Ti)特征峰位置没有变化,说明二者的晶型没有改变,表明催化剂具有良好的稳定性. ...
Photodeposition of metal sulfides on titanium metal-organic frameworks for excellent visible-light-driven photocatalytic Cr(vi) reduction
1
2015
... / mg·L
-1Irradiation | pH | Efficiency /% | Ref. | | Pt/MIL-125-NH2/1000 | Cr(VI)/15 | 300 W Xe lamp | 6 | 75.0% (120 min) | [40] |
| Bi2S3@NH2-MIL-125(Ti)/100 | Cr(VI)/10 | 300 W Xe lamp | 7 | 77.0% (120 min) | [41] |
| CdS/MIL-125(Ti)/500 | Cr(VI)/48 | 300 W Xe lamp(cut~420 nm) | 6 | 35.0% (70 min) | [42] |
| Pd@MIL-101/1000 | Cr(VI)/10 | 125 W light pressure mercury lamp | 6 | 46.4% (240 min) | [43] |
| MIL-125-derived TiO2@C/300 | Cr(VI)/5 | – | 3 | 61.8% (90 min) | [44] |
| Ag3PO4/MIL-125(Ti)-2 | Cr(VI)/10 | 300 W Xe lamp | 6 | 77.6% (90 min) | This work |
| Ag3PO4/MIL-125(Ti)-2 | Cr(VI)/10 | 300 W Xe lamp | 2 | 96.9% (90 min) | This work |
2.4.3 光催化剂的稳定性进行6次光催化循环实验,考察Ag3PO4/MIL-125(Ti)-2的稳定性.图8a表明,4次光催化循环实验后Ag3PO4/MIL-125(Ti)-2的回收率为90.7%,对Cr(VI)的光催化还原率仍保持较高的水平(69.3%).循环次数增至6次后Ag3PO4/MIL-125(Ti)-2对Cr(VI)的光催化还原率由77.6%降低至61.7%,其原因可能是在光催化过程中催化剂损耗.图8b给出了Ag3PO4/MIL-125(Ti)-2在6次光催化反应前后XRD谱的对比.可以看出,经过光催化反应后Ag3PO4/MIL-125(Ti)-2中对应的Ag3PO4和MIL-125(Ti)特征峰位置没有变化,说明二者的晶型没有改变,表明催化剂具有良好的稳定性. ...
Spatial directional separation and synergetic treatment of Cr(VI) and Rhodamine B mixed pollutants on three-layered Pd@MIL-101/P25 photocatalyst
1
2022
... / mg·L
-1Irradiation | pH | Efficiency /% | Ref. | | Pt/MIL-125-NH2/1000 | Cr(VI)/15 | 300 W Xe lamp | 6 | 75.0% (120 min) | [40] |
| Bi2S3@NH2-MIL-125(Ti)/100 | Cr(VI)/10 | 300 W Xe lamp | 7 | 77.0% (120 min) | [41] |
| CdS/MIL-125(Ti)/500 | Cr(VI)/48 | 300 W Xe lamp(cut~420 nm) | 6 | 35.0% (70 min) | [42] |
| Pd@MIL-101/1000 | Cr(VI)/10 | 125 W light pressure mercury lamp | 6 | 46.4% (240 min) | [43] |
| MIL-125-derived TiO2@C/300 | Cr(VI)/5 | – | 3 | 61.8% (90 min) | [44] |
| Ag3PO4/MIL-125(Ti)-2 | Cr(VI)/10 | 300 W Xe lamp | 6 | 77.6% (90 min) | This work |
| Ag3PO4/MIL-125(Ti)-2 | Cr(VI)/10 | 300 W Xe lamp | 2 | 96.9% (90 min) | This work |
2.4.3 光催化剂的稳定性进行6次光催化循环实验,考察Ag3PO4/MIL-125(Ti)-2的稳定性.图8a表明,4次光催化循环实验后Ag3PO4/MIL-125(Ti)-2的回收率为90.7%,对Cr(VI)的光催化还原率仍保持较高的水平(69.3%).循环次数增至6次后Ag3PO4/MIL-125(Ti)-2对Cr(VI)的光催化还原率由77.6%降低至61.7%,其原因可能是在光催化过程中催化剂损耗.图8b给出了Ag3PO4/MIL-125(Ti)-2在6次光催化反应前后XRD谱的对比.可以看出,经过光催化反应后Ag3PO4/MIL-125(Ti)-2中对应的Ag3PO4和MIL-125(Ti)特征峰位置没有变化,说明二者的晶型没有改变,表明催化剂具有良好的稳定性. ...
Intensified redox co-conversion of As(III) and Cr(VI) with MIL-125(Ti)-derived COOH functionalized TiO2: performance and mechanism
1
2019
... / mg·L
-1Irradiation | pH | Efficiency /% | Ref. | | Pt/MIL-125-NH2/1000 | Cr(VI)/15 | 300 W Xe lamp | 6 | 75.0% (120 min) | [40] |
| Bi2S3@NH2-MIL-125(Ti)/100 | Cr(VI)/10 | 300 W Xe lamp | 7 | 77.0% (120 min) | [41] |
| CdS/MIL-125(Ti)/500 | Cr(VI)/48 | 300 W Xe lamp(cut~420 nm) | 6 | 35.0% (70 min) | [42] |
| Pd@MIL-101/1000 | Cr(VI)/10 | 125 W light pressure mercury lamp | 6 | 46.4% (240 min) | [43] |
| MIL-125-derived TiO2@C/300 | Cr(VI)/5 | – | 3 | 61.8% (90 min) | [44] |
| Ag3PO4/MIL-125(Ti)-2 | Cr(VI)/10 | 300 W Xe lamp | 6 | 77.6% (90 min) | This work |
| Ag3PO4/MIL-125(Ti)-2 | Cr(VI)/10 | 300 W Xe lamp | 2 | 96.9% (90 min) | This work |
2.4.3 光催化剂的稳定性进行6次光催化循环实验,考察Ag3PO4/MIL-125(Ti)-2的稳定性.图8a表明,4次光催化循环实验后Ag3PO4/MIL-125(Ti)-2的回收率为90.7%,对Cr(VI)的光催化还原率仍保持较高的水平(69.3%).循环次数增至6次后Ag3PO4/MIL-125(Ti)-2对Cr(VI)的光催化还原率由77.6%降低至61.7%,其原因可能是在光催化过程中催化剂损耗.图8b给出了Ag3PO4/MIL-125(Ti)-2在6次光催化反应前后XRD谱的对比.可以看出,经过光催化反应后Ag3PO4/MIL-125(Ti)-2中对应的Ag3PO4和MIL-125(Ti)特征峰位置没有变化,说明二者的晶型没有改变,表明催化剂具有良好的稳定性. ...
Carbon dot-sensitized urchin-like Ti3+ self-doped TiO2 photocatalysts with enhanced photoredox ability for highly efficient removal of Cr6+ and RhB
1
2020
... 分别用AO、BQ、IPA和K2S2O8作为h+、·O、·OH和e-的捕获剂,测定了光催化反应过程中的活性物种[45].从图9可见,K2S2O8的加入大大降低了Ag3PO4/MIL-125(Ti)-2对Cr(VI)的光催化还原率,说明光生e-在Cr(VI)的还原过程中有决定性作用.加入BQ时Cr(VI)的还原率有所下降,其原因可能是O2/·O介导的还原反应中电子传递降低[4].与之相反的是,加入AO和IPA后Cr(VI)的还原率显著提高,因为光生h+被捕获后与光生e-的分离效率提高,参与还原Cr(VI)的光生e-更多,而·OH被捕获后限制了Cr(III)再次被氧化为Cr(VI). ...
Hierarchical structured ZnFe2O4@SiO2@TiO2 composite for enhanced visible-light photocatalytic activity
1
2018
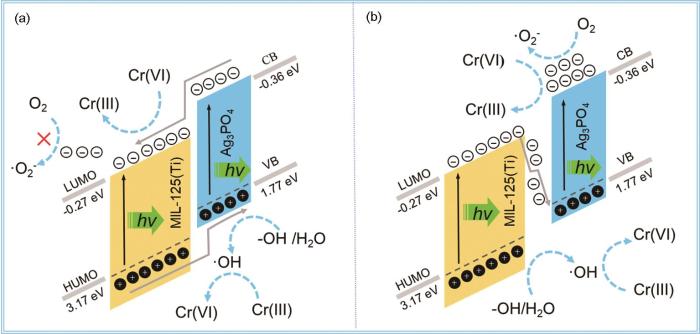
... 根据上述实验结果和分析并结合MIL125(Ti) 和Ag3PO4的能带结构(图3),分析Ag3PO4/MIL-125光催化还原Cr(VI)的机制.图10a表明,在模拟太阳光照射下Ag3PO4和MIL-125均被激发,光生e-分别由Ag3PO4的VB跃迁至CB,由MIL-125(Ti)的HUMO能级跃迁至LUMO能级,而后位于Ag3PO4中CB上的e-转移至MIL-125(Ti)的LUMO能级参与Cr(VI)的还原和·O的形成.位于MIL-125(Ti) HUMO能级上的h+转移至Ag3PO4的VB,进而与H2O/-OH反应生成·OH.但是,由于MIL-125(Ti)的LUMO电位为-0.27 eV,位于其上的e-不能与O2反应生成·O (-0.33 V vs. NHE)[46],这与活性物种捕获实验结果不符.这表明,Ag3PO4/MIL-125(Ti)中的光生e-和h+更可能遵循Z型转移机制.如图10b所示,在模拟太阳光照射下Ag3PO4和MIL-125被激发,位于MIL-125(Ti)的LUMO能级上的光生e-转移至Ag3PO4的VB与其上的h+复合(如 式(5)和(6)),留下位于Ag3PO4的CB上的e-将Cr(VI)还原为Cr(III).同时,e-促进O2转化为·O,·O有助于Cr(VI)的还原(式(7)~(9))[47].同时,·O还可能按照式(10)~(12)的反应参与Cr(III)氧化产生·OH[48,49].而位于MIL-125(Ti)的HUMO能级上的h+与OH-/H2O反应生成·OH,这些·pOH与h+将低价态Cr重新氧化为Cr(VI) (式(13))[50].Ag3PO4和MIL-125(Ti)形成的Z型异质结使光生e-和h+的分离效率提高,加快光了催化还原Cr(VI)的进程. ...
Ag NPs decorated C-TiO2/Cd0.5Zn0.5S Z-scheme heterojunction for simultaneous RhB degradation and Cr(VI) reduction
1
2021
... 根据上述实验结果和分析并结合MIL125(Ti) 和Ag3PO4的能带结构(图3),分析Ag3PO4/MIL-125光催化还原Cr(VI)的机制.图10a表明,在模拟太阳光照射下Ag3PO4和MIL-125均被激发,光生e-分别由Ag3PO4的VB跃迁至CB,由MIL-125(Ti)的HUMO能级跃迁至LUMO能级,而后位于Ag3PO4中CB上的e-转移至MIL-125(Ti)的LUMO能级参与Cr(VI)的还原和·O的形成.位于MIL-125(Ti) HUMO能级上的h+转移至Ag3PO4的VB,进而与H2O/-OH反应生成·OH.但是,由于MIL-125(Ti)的LUMO电位为-0.27 eV,位于其上的e-不能与O2反应生成·O (-0.33 V vs. NHE)[46],这与活性物种捕获实验结果不符.这表明,Ag3PO4/MIL-125(Ti)中的光生e-和h+更可能遵循Z型转移机制.如图10b所示,在模拟太阳光照射下Ag3PO4和MIL-125被激发,位于MIL-125(Ti)的LUMO能级上的光生e-转移至Ag3PO4的VB与其上的h+复合(如 式(5)和(6)),留下位于Ag3PO4的CB上的e-将Cr(VI)还原为Cr(III).同时,e-促进O2转化为·O,·O有助于Cr(VI)的还原(式(7)~(9))[47].同时,·O还可能按照式(10)~(12)的反应参与Cr(III)氧化产生·OH[48,49].而位于MIL-125(Ti)的HUMO能级上的h+与OH-/H2O反应生成·OH,这些·pOH与h+将低价态Cr重新氧化为Cr(VI) (式(13))[50].Ag3PO4和MIL-125(Ti)形成的Z型异质结使光生e-和h+的分离效率提高,加快光了催化还原Cr(VI)的进程. ...
Synergistic photocatalysis of Cr(VI) reduction and 4-Chlorophenol degradation over hydroxylated α-Fe2O3 under visible light irradiation
1
2016
... 根据上述实验结果和分析并结合MIL125(Ti) 和Ag3PO4的能带结构(图3),分析Ag3PO4/MIL-125光催化还原Cr(VI)的机制.图10a表明,在模拟太阳光照射下Ag3PO4和MIL-125均被激发,光生e-分别由Ag3PO4的VB跃迁至CB,由MIL-125(Ti)的HUMO能级跃迁至LUMO能级,而后位于Ag3PO4中CB上的e-转移至MIL-125(Ti)的LUMO能级参与Cr(VI)的还原和·O的形成.位于MIL-125(Ti) HUMO能级上的h+转移至Ag3PO4的VB,进而与H2O/-OH反应生成·OH.但是,由于MIL-125(Ti)的LUMO电位为-0.27 eV,位于其上的e-不能与O2反应生成·O (-0.33 V vs. NHE)[46],这与活性物种捕获实验结果不符.这表明,Ag3PO4/MIL-125(Ti)中的光生e-和h+更可能遵循Z型转移机制.如图10b所示,在模拟太阳光照射下Ag3PO4和MIL-125被激发,位于MIL-125(Ti)的LUMO能级上的光生e-转移至Ag3PO4的VB与其上的h+复合(如 式(5)和(6)),留下位于Ag3PO4的CB上的e-将Cr(VI)还原为Cr(III).同时,e-促进O2转化为·O,·O有助于Cr(VI)的还原(式(7)~(9))[47].同时,·O还可能按照式(10)~(12)的反应参与Cr(III)氧化产生·OH[48,49].而位于MIL-125(Ti)的HUMO能级上的h+与OH-/H2O反应生成·OH,这些·pOH与h+将低价态Cr重新氧化为Cr(VI) (式(13))[50].Ag3PO4和MIL-125(Ti)形成的Z型异质结使光生e-和h+的分离效率提高,加快光了催化还原Cr(VI)的进程. ...
Simultaneous photocatalytic Cr(VI) reduction and 2,4,6-TCP oxidation over g-C3N4 under visible light irradiation
1
2014
... 根据上述实验结果和分析并结合MIL125(Ti) 和Ag3PO4的能带结构(图3),分析Ag3PO4/MIL-125光催化还原Cr(VI)的机制.图10a表明,在模拟太阳光照射下Ag3PO4和MIL-125均被激发,光生e-分别由Ag3PO4的VB跃迁至CB,由MIL-125(Ti)的HUMO能级跃迁至LUMO能级,而后位于Ag3PO4中CB上的e-转移至MIL-125(Ti)的LUMO能级参与Cr(VI)的还原和·O的形成.位于MIL-125(Ti) HUMO能级上的h+转移至Ag3PO4的VB,进而与H2O/-OH反应生成·OH.但是,由于MIL-125(Ti)的LUMO电位为-0.27 eV,位于其上的e-不能与O2反应生成·O (-0.33 V vs. NHE)[46],这与活性物种捕获实验结果不符.这表明,Ag3PO4/MIL-125(Ti)中的光生e-和h+更可能遵循Z型转移机制.如图10b所示,在模拟太阳光照射下Ag3PO4和MIL-125被激发,位于MIL-125(Ti)的LUMO能级上的光生e-转移至Ag3PO4的VB与其上的h+复合(如 式(5)和(6)),留下位于Ag3PO4的CB上的e-将Cr(VI)还原为Cr(III).同时,e-促进O2转化为·O,·O有助于Cr(VI)的还原(式(7)~(9))[47].同时,·O还可能按照式(10)~(12)的反应参与Cr(III)氧化产生·OH[48,49].而位于MIL-125(Ti)的HUMO能级上的h+与OH-/H2O反应生成·OH,这些·pOH与h+将低价态Cr重新氧化为Cr(VI) (式(13))[50].Ag3PO4和MIL-125(Ti)形成的Z型异质结使光生e-和h+的分离效率提高,加快光了催化还原Cr(VI)的进程. ...
The facile fabrication of 2D/3D Z-scheme g-C3N4/UiO-66 heterojunction with enhanced photocatalytic Cr(VI) reduction performance under white light
1
2019
... 根据上述实验结果和分析并结合MIL125(Ti) 和Ag3PO4的能带结构(图3),分析Ag3PO4/MIL-125光催化还原Cr(VI)的机制.图10a表明,在模拟太阳光照射下Ag3PO4和MIL-125均被激发,光生e-分别由Ag3PO4的VB跃迁至CB,由MIL-125(Ti)的HUMO能级跃迁至LUMO能级,而后位于Ag3PO4中CB上的e-转移至MIL-125(Ti)的LUMO能级参与Cr(VI)的还原和·O的形成.位于MIL-125(Ti) HUMO能级上的h+转移至Ag3PO4的VB,进而与H2O/-OH反应生成·OH.但是,由于MIL-125(Ti)的LUMO电位为-0.27 eV,位于其上的e-不能与O2反应生成·O (-0.33 V vs. NHE)[46],这与活性物种捕获实验结果不符.这表明,Ag3PO4/MIL-125(Ti)中的光生e-和h+更可能遵循Z型转移机制.如图10b所示,在模拟太阳光照射下Ag3PO4和MIL-125被激发,位于MIL-125(Ti)的LUMO能级上的光生e-转移至Ag3PO4的VB与其上的h+复合(如 式(5)和(6)),留下位于Ag3PO4的CB上的e-将Cr(VI)还原为Cr(III).同时,e-促进O2转化为·O,·O有助于Cr(VI)的还原(式(7)~(9))[47].同时,·O还可能按照式(10)~(12)的反应参与Cr(III)氧化产生·OH[48,49].而位于MIL-125(Ti)的HUMO能级上的h+与OH-/H2O反应生成·OH,这些·pOH与h+将低价态Cr重新氧化为Cr(VI) (式(13))[50].Ag3PO4和MIL-125(Ti)形成的Z型异质结使光生e-和h+的分离效率提高,加快光了催化还原Cr(VI)的进程. ...