各向异性金纳米棒,是近年来研究得较为深入的一种贵金属纳米颗粒。目前,制备金纳米棒的主要方法有模板法[11]、光诱导法[12]、电化学法[13]和种子生长法[14]。模板法使用合适的模板制备目标纳米颗粒,但是产率较低且制备过程繁杂。光诱导法是在使用特定的反应溶剂条件下对生长溶液进行不同波长的光辐射,但是生长的金纳米棒形貌不均一且稳定性较差。电化学法是对反应溶液施加电场制备金纳米棒,但是重复性不高、合成工序复杂且不可控。在种子生长法中颗粒的成核和生长分别进行,使产物的产率、形貌均匀性比较高[15]。使用种子生长法合成金纳米棒,可通过改变生长液中各反应参量调控其形貌和尺寸。鉴于此,本文研究生长液中AgNO3、CTAB、籽晶用量对金纳米棒形貌及产率的影响,优化反应条件以合成形貌均一、产率高、分散性好、重复性高的金纳米棒。
1 实验方法
1.1 金种子的制备
用分析天平(型号为PX84ZH)称取0.3553 g (0.1 mol/L)的十六烷基三甲基溴化铵(CTAB,分析纯)溶于9.75 mL的纯水中,然后将浓度为0.01 mol/L的氯酸金(HAuCl4·3H2O,分析纯)溶液0.25 mL与上述溶液充分混合呈黄色溶液,在用磁力加热搅拌器(型号为85-2型)剧烈搅拌下快速加入冰水现配的0.01 mol/L硼氢化钠(NaBH4,分析纯)溶液0.6 mL,溶液颜色由黄色变为茶棕色表明金种子形成,搅拌2 min后得到金种溶液,将其在室温下静置2-4 h得到金种子。金种子制备流程,如图1所示。
图1
图1
制备晶种溶液的流程图
Fig.1
A Schematic diagram for the preparation of gold seed solution
1.2 金纳米棒的合成
制备长径比为3.8的小型金纳米棒。用用分析天平称取0.328 g (0.1 mol/L)的CTAB溶于9 mL的纯水中,然后分别加入0.01 mol/L的HAuCl4溶液0.5 mL、0.01 mol/L的硝酸银(AgNO3,分析纯)溶液0.1 mL以及1 mol/L的HCl溶液0.2 mL,使其混合均匀后加入0.1 mol/L的抗坏血酸(AA,分析纯)溶液0.08 mL,溶液的颜色由深黄色变为无色。用磁力加热搅拌器搅拌2 min后快速加入1 mL种子溶液。将所得溶液在室温下静置过夜,制备过程如图2所示。将最终得到的溶液用离心机(型号为HC-2518)以12000的转速离心30 min,弃掉上清液后加入一定量的纯水重新分散,离心分离两次后得到金纳米棒。
图2
图2
制备生长溶液的流程图
Fig.2
A schematic illustration of the synthesis of growth solution
1.3 金纳米棒的表征和对福美双的检测
用分光光度计(型号为Lambda950)测试金纳米棒的吸收光谱;用透射电子显微镜(型号为JEM2010)观察金纳米棒的形貌;用制得的金纳米棒作为增强基底用于检测农药残留福美双(Thiram,分析纯)。用一次性针管吸取金纳米棒溶液,滴一滴到面积为5 mm×5 mm的干净玻璃片上,使其自然干燥。然后用分析天平称取12 mg的福美双粉末,用无水乙醇作溶剂配制成浓度为10-2 mol/L的福美双溶液。然后用无水乙醇依次稀释成浓度为10-3 mol/L-10-7 mol/L的待测溶液。最后将不同浓度的福美双溶液分别滴到上述玻璃片上,待溶剂干燥后使用激光拉曼光谱仪(型号为HR Evolution)进行SERS检测。拉曼测试的激发波长为785 nm,使用50倍物镜,积分时间为10 s,扫描次数为2次,功率约为3 mW。
2 结果和讨论
2.1 AgNO3 的用量对金纳米棒的影响
图3
图3
AgNO3(0.035、0.065、0.08、0.1 mL)用量不同的金纳米棒的吸收光谱
Fig.3
Absorption spectra of gold nanorods prepared with different amounts (0.035、0.065、0.08、0.1 mL) of AgNO3
图4
图4
AgNO3(a: 0.035 mL、b: 0.065 mL、c: 0.08 mL、d: 0.1 mL)用量不同的金纳米棒的TEM照片
Fig.4
TEM images of gold nanorods synthesized by different contents (a: 0.035 mL、b: 0.065 mL、c: 0.08 mL、d: 0.1 mL) of AgNO3 (Scale bars: 100 nm)
表1 AgNO3用量对金纳米棒平均长度、直径、长径比、纵向共振峰波长和产率的影响
Table 1
| 0.01 mol/L AgNO3 /mL | Length /nm | Diameter /nm | Aspect ratio (R=L/D) | Longitudinal SPR /nm | Yield /% |
|---|---|---|---|---|---|
| 0.035 | 44.5 | 10.5 | 4.2 | 857 | 96 |
| 0.065 | 39.1 | 9.6 | 4.1 | 848 | 98 |
| 0.08 | 38.2 | 9.7 | 3.9 | 832 | 94 |
| 0.1 | 32.9 | 8.9 | 3.7 | 813 | 90 |
2.2 籽晶的影响
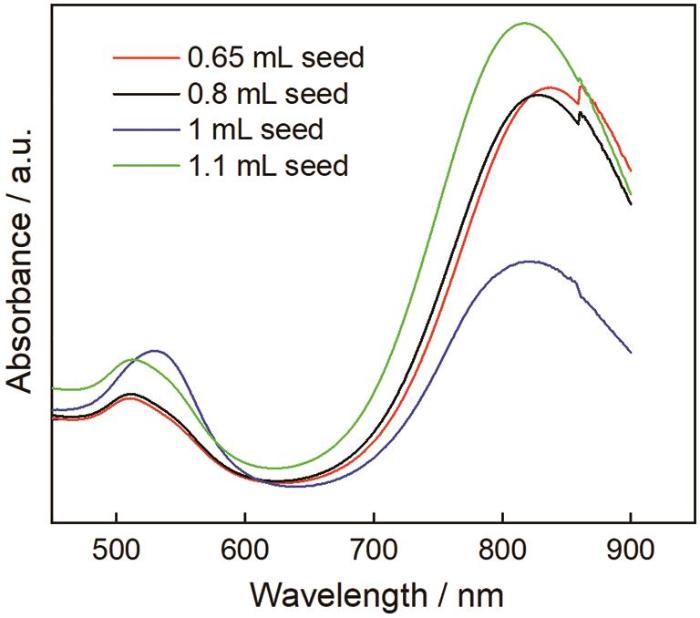
籽晶是金纳米棒定向生长的“核”[19]。图5给出了籽晶用量分别为0.65 mL、0.8 mL、1 mL和1.1 mL制备的金纳米棒的吸收光谱。可以看出,籽晶量为0.65、0.8和1.1 mL制备的金纳米棒,其吸收光谱中横向共振吸收峰位置都约为511 nm,而纵向共振吸收峰位从855 nm减小至809 nm,峰的分布瘦而尖但是强度大。这表明,金纳米棒的长径比没有明显变化,主要产物是金纳米棒。在籽晶用量为1 mL的吸收光谱中,出现位于530 nm的横向共振吸收峰和位于814 nm处的纵向共振吸收峰,且纵向共振吸收峰峰型矮而宽。这表明,最终产物中有其它形貌的副产颗粒,金纳米棒的产率不高。由此可见,只有籽晶的用量适当才能提高金纳米棒的产率,减少副产颗粒。
图5
图5
籽晶(0.65 mL、0.8 mL、1 mL、1.1 mL)用量不同的金纳米棒的吸收光谱
Fig.5
Absorption spectra of gold nanorods prepared with different amounts (0.65 mL、0.8 mL、1 mL、1.1 mL) of seed
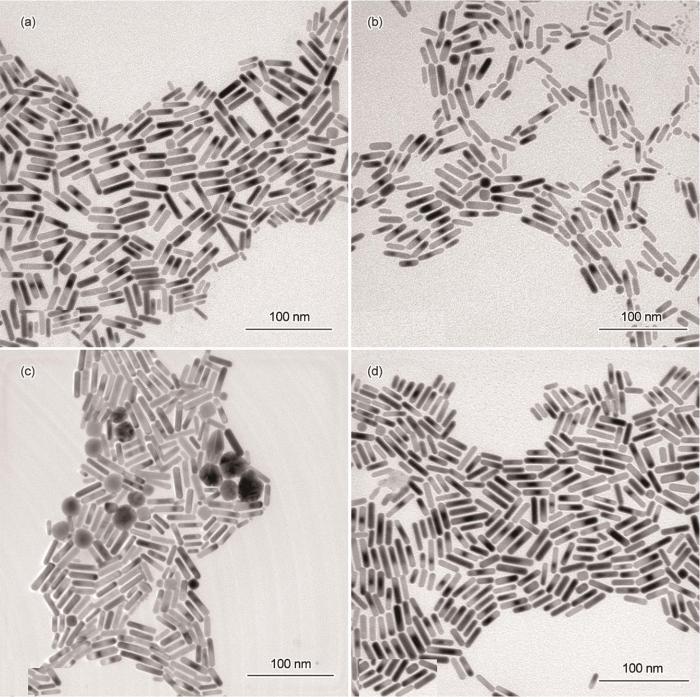
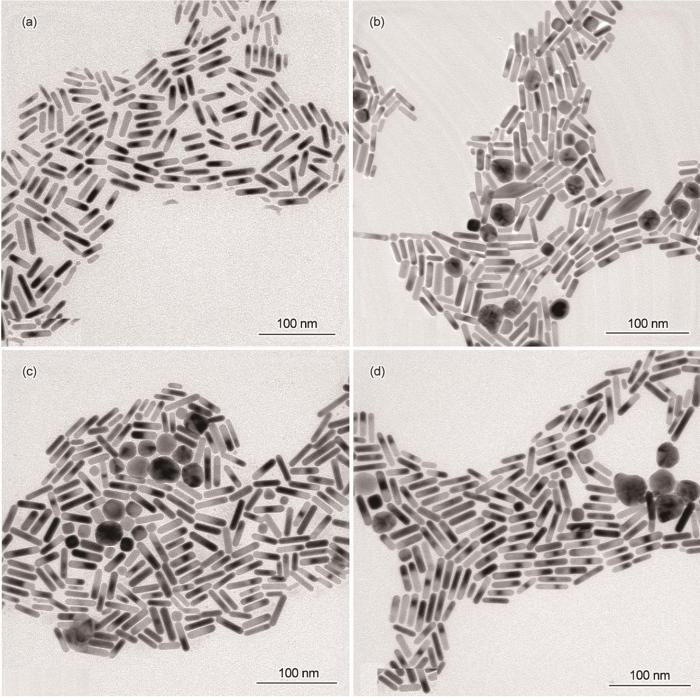
图6给出了籽晶用量不同的金纳米棒的TEM照片。从图6可见,籽晶加入量为0.65 mL时的产物大多是金纳米棒,尺寸分布较为均一,长度约为39.6 nm,只有微量的球形颗粒(图6a)。籽晶加入量为0.8 mL时合成的金纳米棒直径较为一致,尺寸有长有短,形貌的均匀性略差(图6b)。籽晶的用量为1 mL时产物中出现一些大小不一的球形颗粒,球形颗粒明显增多,金纳米棒的产率降低(~90%)(图6c)。在图5的吸收光谱中,位于820 nm的纵向共振吸收峰强度与其它谱线相差较大,且两端分布较宽,从而证实了这一现象。继续加大籽晶量至1.1 mL时金纳米棒的产率明显提高(~98%),且形貌均匀性、分散性更好,几乎没有副产颗粒(图6d)。这与图5吸收光谱中纵向共振吸收峰的中间细,两端窄的情况一致。这表明,籽晶用量对金纳米棒的形貌及产率有显著的影响。生长液中籽晶不够使金纳米棒的产率降低;而籽晶用量过高则产物中出现副产颗粒。籽晶用量适当,有利于制备出高产率、高均一性的金纳米棒。
图6
图6
籽晶(a: 0.65 mL、b: 0.8 mL、c: 1 mL、d: 1.1 mL)用量不同的金纳米棒的TEM照片
Fig.6
TEM images of gold nanorods synthesized by different contents (a: 0.65 mL、b: 0.8 mL、c: 1 mL、d: 1.1 mL) of seed (Scale bars: 100 nm)
根据不同籽晶量制备的金纳米棒粒径的分布,计算长径比,结果列于表2。可以看出,当体系中其它反应物用量和浓度一定时,金纳米棒的长径比与籽晶用量之间存在反比关系。随着籽晶量的增加其长径比明显减小,纵向共振吸收峰波长发生蓝移。这表明,对于一定数量的金原子,籽晶用量的增加使提供给每个籽晶的金原子数量减少,导致最终产物中出现副产颗粒。
表2 籽晶用量对金纳米棒平均长度、直径、长径比、纵向共振峰波长和产率的影响
Table 2
Au seed /mL | Length /nm | Diameter /nm | Aspect ratio (R=L/D) | Longitudinal SPR /nm | Yield /% |
|---|---|---|---|---|---|
| 0.65 | 39.6 | 9.5 | 4.2 | 855 | 99 |
| 0.8 | 38.5 | 9.8 | 3.9 | 838 | 95 |
| 1 | 32.7 | 8.9 | 3.7 | 814 | 90 |
| 1.1 | 29.9 | 8.3 | 3.6 | 809 | 98 |
2.3 CTAB的影响
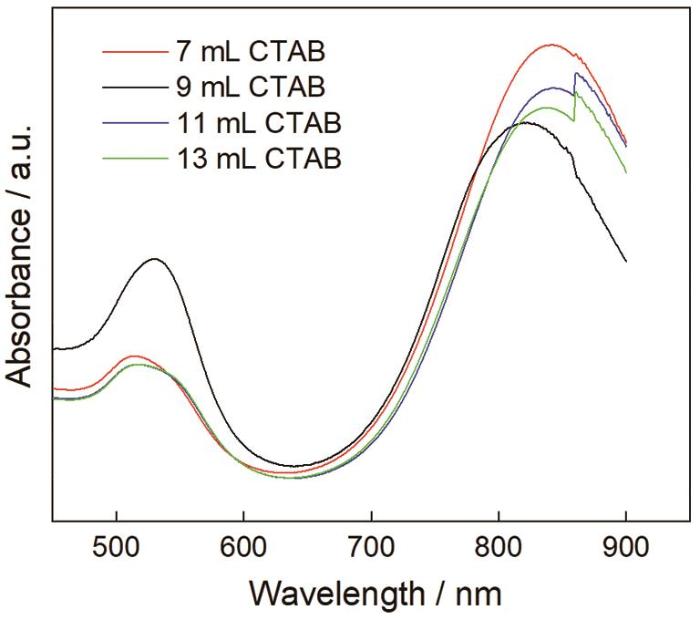
在金纳米棒的合成过程中,表面活性剂CTAB以双分子层形式择优吸附在金纳米棒表面的不同晶面,从而控制金纳米棒的生长[20]。图7给出了用浓度为0.1 mol/L不同量的CTAB合成的金纳米棒的吸收光谱。从图7可以看出,4种金纳米棒的吸收光谱十分相似,且横向共振吸收峰位基本不变,而纵向共振吸收峰分别出现在815 nm、822 nm、837 nm及843 nm。峰值变化不明显,说明金纳米棒的长径比几乎保持一致。CTAB用量进一步增大至11和13 mL,纵向共振吸收峰发生变化,两者均在859 nm处出现拐点。其原因是,CTAB用量过大使体系中Br‒离子的强度提高。大量吸附在金纳米棒表面的Br‒离子抑制了金纳米棒的定向生长,降低了金纳米棒的产率,并产生副产颗粒[21]。
图7
图7
CTAB(7 mL、9 mL、11 mL、13 mL)用量不同的金纳米棒的吸收光谱
Fig.7
Absorption spectra of gold nanorods prepared with different amounts (7 mL、9 mL、11 mL、13 mL) of CTAB
在Au3+、AgNO3、籽晶和抗坏血酸的用量及浓度一定时,改变CTAB用量制备的金纳米棒,其尺寸分布在图8中给出。从图8中的金纳米棒透射电镜照片可以看出,加入7 mL的CTAB时金纳米棒的形貌均一性、分散性良好,且直径较小,长径比约为3.7,平均长度约为33.9 nm(图8a)。这个结果,与图7吸收光谱中位于841 nm处的纵向共振吸收峰强度高于其它曲线且峰型瘦而尖相对应。加入9 mL的CTAB时产物中出现较多的不规则金纳米颗粒,金纳米棒的产率和形貌均匀性都不理想(图8b)。这表明,生长液中过量的CTAB不利于金纳米棒的生长。继续增加CTAB用量至11 mL(图8c)和13 mL(图8d)时,产物大多为金纳米棒和少量的大小不一的球形颗粒。金纳米棒两端类似于椭圆形,表面光滑和分散性良好,其平均长度约为35.5 nm和36.6 nm,长径比大多为3.9。
图8
图8
CTAB(a: 7 mL、b: 9 mL、c: 11 mL、d: 13 mL)用量不同的金纳米棒的TEM照片
Fig.8
TEM images of gold nanorods synthesized by different contents (a: 7 mL、b: 9 mL、c: 11 mL、d: 13 mL) of CTAB (Scale bars: 100 nm)
表3 CTAB用量对金纳米棒平均长度、直径、长径比、纵向共振峰波长和产率的影响
Table 3
0.1 mol/L CTAB /mL | Length /nm | Diameter /nm | Aspect ratio (R=L/D) | Longitudinal SPR /nm | Yield /% |
|---|---|---|---|---|---|
| 7 | 33.9 | 9.2 | 3.7 | 815 | 96 |
| 9 | 32.7 | 8.6 | 3.8 | 822 | 87 |
| 11 | 35.5 | 9.1 | 3.9 | 837 | 89 |
| 13 | 36.6 | 9.3 | 3.9 | 843 | 93 |
2.4 在优化条件下制备的金纳米棒的光谱和形貌
图9
图9
在优化条件下制备的金纳米棒的吸收光谱
Fig.9
Absorption spectrum of gold nanorods prepared under optimized conditions
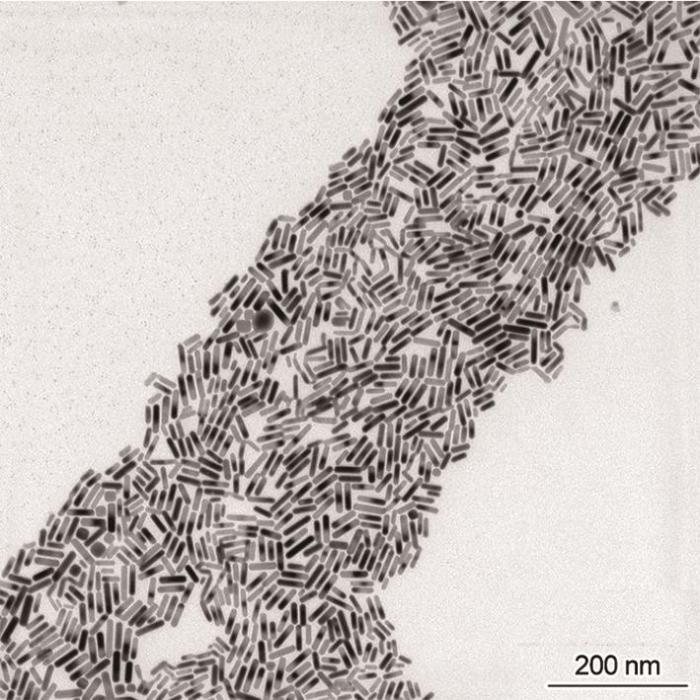
图10
图10
在优化条件下制备的金纳米棒的TEM照片
Fig.10
TEM images of gold nanorods prepared under optimized conditions
图11
2.5 小型金纳米棒对福美双的拉曼光谱
图12a给出了用金纳米棒检测不同浓度福美双的拉曼增强光谱。可以看出,在金纳米棒的增强谱线中均出现了明显的福美双特定的拉曼特征,分别位于552,1142,1375,1499 cm-1处。为了分析SERS强度与福美双浓度的关系,以1374 cm-1处的特征峰强度为依据,研究福美双的浓度对SERS增强性能的影响。由图12b可见,随着福美双的浓度由10-7 mol/L提高到10-2 mol/L,其特征峰的强度显著提高。这表明,随着福美双浓度的提高金纳米棒基底的SERS活性随之提高,能检测到的福美双最低浓度达10-7 mol/L,低于规定的各食品中最大残留限值。与其它功能化金纳米棒基底对福美双的SERS检测体系相比[22,23],此法更加简单可控且稳定性高。
图12
图12
用金纳米棒检测福美双的拉曼增强光谱以及1374 cm-1处的特征峰强度与福美双浓度的关系
Fig.12
Roman enhanced spectrum of different concentrations of thiram solution on gold nanorods substrate (a) and the relationship between the intensity of the 1374 cm-1 peak and thiram concentrations (b)
3 结论
(1) 用种子生长法可制备金纳米棒。随着AgNO3用量的增加Ag+引导金种定向生长成稳定的棒状结构,制备出的金纳米棒形貌均一。籽晶的用量过少不能为金纳米棒的生长提供充足的金种,使金纳米棒的产率降低。CTAB用量过大则难以控制金纳米棒不同晶面的生长速度,使金纳米棒中出现较多不规则的球形颗粒。
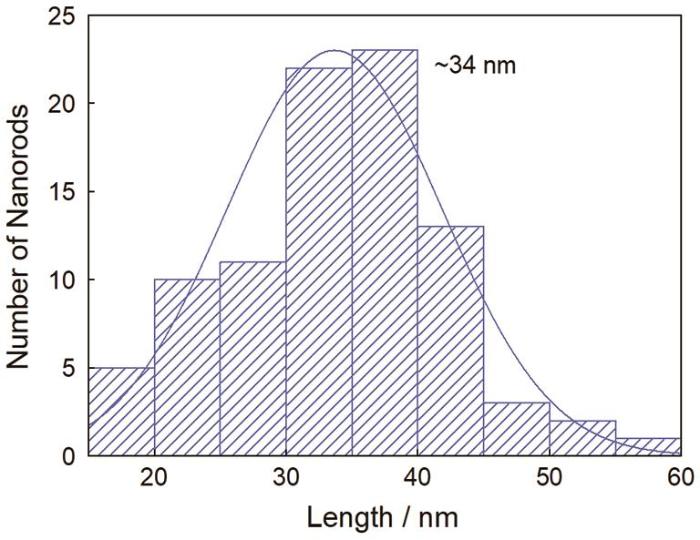
(2) 种子生长法的优化条件:(0.01 mol/L) AgNO3用量为0.035 mL,(0.1 mol/L) CTAB用量为11 mL,籽晶用量为1.1 mL。在优化条件下制备出的金纳米棒形貌一致、分散性好,长径比约为3.8,平均长度约为34 nm。
(3) 这种小尺寸金纳米棒具有较大的吸收截面和更高的光热效率,可用于检测低浓度生物分子。
参考文献
Surface plasmon subwavelength optics:principles and novel effects
[J].
表面等离子体亚波长光学原理和新颖效应
[J].
Structural and electronic properties of micellar Au nanoparticles: size and ligand effects
[J].Gaining experimental insight into the intrinsic properties of nanoparticles (NPs) represents a scientific challenge due to the difficulty of deconvoluting these properties from various environmental effects such as the presence of adsorbates or a support. A synergistic combination of experimental and theoretical tools, including X-ray absorption fine-structure spectroscopy, scanning transmission electron microscopy, atomic force microscopy, and density functional theory was used in this study to investigate the structure and electronic properties of small (∼1-4 nm) Au NPs synthesized by an inverse micelle encapsulation method. Metallic Au NPs encapsulated by polystyrene 2-vinylpiridine (PS-P2VP) were studied in the solution phase (dispersed in toluene) as well as after deposition on γ-Al2O3. Our experimental data revealed a size-dependent contraction of the interatomic distances of the ligand-protected NPs with decreasing NP size. These findings are in good agreement with the results from DFT calculations of unsupported Au NPs surrounded by P2VP, as well as those obtained for pure (ligand-free) Au clusters of analogous sizes. A comparison of the experimental and theoretical results supports the conclusion that the P2VP ligands employed to stabilize the gold NPs do not lead to strong distortions in the average interatomic spacing. The changes in the electronic structure of the Au-P2VP NPs were found to originate mainly from finite size effects and not from charge transfer between the NPs and their environment (e.g., Au-ligand interactions). In addition, the isolated ligand-protected experimental NPs only display a weak interaction with the support, making them an ideal model system for the investigation of size-dependent physical and chemical properties of structurally well-defined nanomaterials.
Nanostructure-based plasmon-enhanced raman spectroscopy for surface analysis of materials
[J].
A quantitative study of the environmental effects on the optical response of gold nanorods
[J].The effects of the dielectric environment on the optical extinction spectra of gold nanorods were quantitatively studied using individual bare and silica-coated nanorods. The dispersion and amplitude of their extinction cross-section, dominated by absorption for the investigated sizes, were measured using spatial modulation spectroscopy (SMS). The experimental results were compared to calculations from a numerical model that included environmental features present in the measurements and the morphology and size of the corresponding nanorods measured by transmission electron microscopy. The combination of these experimental and theoretical tools permits a detailed interpretation of the optical properties of the individual nanorods. The measured optical extinction spectra and the extinction cross-section amplitudes were well reproduced by the numerical model for silica-coated gold nanorods, for which the silica shell provides a controlled environment. In contrast, additional environmental factors had to be assumed in the model for bare nanorods, stressing the importance of controlling and characterizing the experimental conditions when measuring the optical response of bare surface-deposited single metal nanoparticles.
Targeting cancer cell integrins using gold nanorods in photothermal therapy inhibits migration through affecting cytoskeletal proteins
[J].
Targeting nano drug delivery to cancer cells using tunable, multi-layer, silver-decorated gold nanorods
[J].
Engineered silver nanoparticle (Ag-NP) behaviour in domestic on-site wastewater treatment plants and in sewage sludge amended-soils
[J].
Tip-specific functionalization of gold nanorods for plasmonic biosensing: effect of linker chain length
[J].
A single-crystalline silver plasmonic circuit for visible quantum emitters
[J].
Surface-enhanced raman spectroscopy: concepts and chemical applications
[J].
Templated growth of surface enhanced raman scattering-active branched gold nanoparticles within radial mesoporous silica shells
[J].Noble metal nanoparticles are widely used as probes or substrates for surface enhanced Raman scattering (SERS), due to their characteristic plasmon resonances in the visible and near-IR spectral ranges. Aiming at obtaining a versatile system with high SERS performance, we developed the synthesis of quasi-monodisperse, nonaggregated gold nanoparticles protected by radial mesoporous silica shells. The radial mesoporous channels were used as templates for the growth of gold tips branching out from the cores, thereby improving the plasmonic performance of the particles while favoring the localization of analyte molecules at high electric field regions: close to the tips, inside the pores. The method, which additionally provides control over tip length, was successfully applied to gold nanoparticles with various shapes, leading to materials with highly efficient SERS performance. The obtained nanoparticles are stable in ethanol and water upon thermal consolidation and can be safely stored as a powder.
Nanorod formation by photochemical metal deposition in nanoporous aluminum oxide templates
[J].
Electrochemical synthesis of gold nanorods in track-etched polycarbonate membrane using removable mercury cathode
[J].
Mini gold nanorods with tunable plasmonic peaks beyond 1000 nm
[J].
Gold nanorods: synthesis, growth mechanism and purification
[J].
金纳米棒的制备、生长机理及纯化
[J].
Wet chemical synthesis of high aspect ratio cylindrical gold nanorods
[J].
Preparation and growth mechanism of gold nanorods (NRs) using seed-med -iated growth method
[J].
Synthesis of absorption-dominant small gold nanorods and their plasmonic properties
[J].
Seed-mediated synthesis of size-tunable and monodisperse gold nanorods through the use of anionic surfactant as additives
[J].
阴离子表面活性剂作为添加剂种子生长法制备尺寸可调的单分散金纳米棒
[J].
Polyvinylpyrrolidone(PVP) in nanoparticle synthesis
[J].
A novel method to prepare high-aspect ratio gold nanorods
[J].A<strong> </strong>novel approach to high-aspect ratio gold nanorods has been presented. The gold nanorods as long as (200±18.62) nm with aspect ratio of above 10 were prepared with optimizing the concentration of surfactant cetyltrimethylammonium bromide (CTAB) by seed-mediated growth method at 25 ℃. Mechanism for gold nanorods formation is discussed. It is shown that the aspect ratio and longitudinal surface plasmon resonance (SPR) can be correlated with the concentration of CTAB. Moreover, by simply enhancing the ion strength of the reaction solution, the as-prepared gold nanorods can be purified for the different electrostatic aggregation effects between gold nanorods and spherical nanoparticles. Shape change of gold nanorods is confirmed by transmission electron microscopy images (TEM) and scan electron microscopy (SEM).
一种制备高长径比金纳米棒的新方法
[J].
On the use of Au@Ag core-shell nanorods for SERS detection of thiram diluted solutions
[J].
Gold nanorod-coated capillaries for the SERS-based detection of thiram
[J].Surface-enhanced Raman scattering (SERS)based capillary system is a promising route toward fast, real-time, and in-situ detection using a facile sampling process. Here, we demonstrate for the first time resonance-tunable SERS-active capillaries with high sensitivity, reproducibility, and stability. The strong signal consistency independent of measurement spots or storage time supports the long-term storage and signal tracking of analytes in practical use. The capillaries were successfully applied to the in-situ detection of pesticide residues, and the sampling process provides operation conveniency compared to conventional methods. These results indicate that our SERS-active capillaries have great potentials in fast in-situ detection for many practical applications.