抗生素广泛应用于医疗、畜牧业、水产业等领域,排放或残留在水或土壤中对生态环境和人类健康构成严重威胁[1~3]。光催化氧化技术,是去除水体中抗生素的一种重要手段[4,5]。光催化材料的吸收系数决定其吸光性能,掺杂可调控其光吸收性能[6]。Hu等[7]用金属或非金属掺杂将TiO2的吸收光谱扩展到可见光范围;Xie等[8]在In2S3原子层中掺杂Co离子引入杂质能级使其载流子浓度提高,极大地提高了材料的可见光吸收性能;Huang等[9]用CO32-自掺杂Bi2O2CO3形成掺杂能级使其导带降低,提高了光催化净化NO的性能。翟等[10]用Cu掺杂TiO2在禁带中形成掺杂能级减小其带隙,利用可见光提高光催化合成氨的性能。
1 材料制备和计算方法
1.1 Li2Sn1-x Si x O3(x=0,0.05,0.1)的制备
采用固相烧结法制备Li2Sn1-x Si x O3(x=0,0.05,0.1)。以Li2Sn1-x Si x O3(x=0.1)为例。用玛瑙研钵将3.3 mmol的碳酸锂(Li2CO3,纯度99.90%)2.70 mmol 的二氧化锡(SnO2,纯度99.5%)和0.30 mmol的二氧化硅(SiO2,纯度98.5%)研磨30 min后置于坩埚,然后放入温度为850℃的高温马弗炉(型号XL-6A)中烧结烧结10 h,烧结结束后自然冷却至室温。取出样品将其研磨10 min后进行二次烧结,烧结温度为850℃,时间为10 h,烧结结束后自然冷却至室温。用相同的方法制备其余样品,得到一系列催化剂。样品的编号:Li2SnO3记为LSO,Li2Sn0.95Si0.05O3记为LSSO-5,Li2Sn0.90Si0.10O3记为LSSO-10。
1.2 光催化剂的表征
使用X射线衍射仪(岛津XRD—7000)测试粉末样品的XRD谱,Cu Kα靶(40 kV和40 mA),步长为0.0262°,每步时长30 S,扫描范围10~80°;用紫外分光光度计(岛津UV-2550)测试四环素盐酸盐(TC,纯度99.90%)溶液的紫外可见吸收光谱;用JEOL JSM-6700F场发射扫描电子显微镜观察材料的表面结构。
在500 W汞灯(型号:CEL-M500)紫外光的照射下,以TC为模拟污染物来评价Li2Sn1-x Si x O3(x=0,0.05,0.1)的光催化活性。在光催化反应过程中,反应液面离光源的距离为20 cm,光功率密度为100 mW/cm2。先将30 mg光催化剂Li2Sn1-x Si x O3放入浓度为20 mg/L的TC溶液(100 mL)中,进行暗吸附60 min使其达到吸附与脱附平衡。然后开启光源,每隔5 min取5 mL上清液,使用离心机(型号:TG-16)将其离心分离后在TC的最大吸收波长357 nm处测其吸光度。以溶液的降解率C/C0=A/A0(C和C0分别为光照后溶液的浓度和初始溶液的浓度,A和A0分别为光照后溶液的吸光度和初始溶液的吸光度)评价光催化剂的活性。
自由基捕获实验:加入10 mL的异丙醇(IPA,纯度99.7%),0.1 mmol 的2,2,6,6-四甲基哌啶醇(TEMPO,纯度98%)和0.1 mmol的乙二胺四乙酸(EDTA,纯度99.5%)分别检测·OH、
1.3 理论计算方法
2 结果和讨论
2.1 物相分析
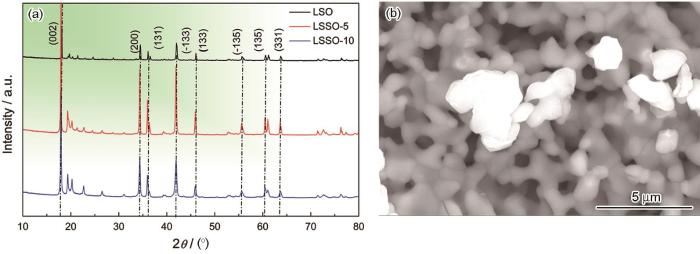
图1a给出了Li2Sn1-x Si x O3(x=0,0.05,0.10)的XRD谱。Li2SnO3为单斜相,其空间群为C2/c。从衍射谱中角度为17.950°,34.413°,35.961°,41.883°,45.905°,55.526°,60.991°和63.657°的衍射峰对应Li2SnO3的晶面分别为(002),(200),(131),(-133),(133),(-135),(135)和(331)[19]。Si掺杂的样品与母体Li2SnO3保持一致。晶胞参数计算结果(表1)表明,随着Si掺杂量的增加晶胞体积呈下降趋势。图1b给出了Li2SnO3的扫描电镜图片,可见用固相烧结法制备的Li2SnO3呈无规则块状且其尺寸大小不均一,颗粒的平均尺寸为1.32 µm。
图1
图1
Li2Sn1-x Si x O3(x=0,0.05,0.1)的XRD谱(a)和LSO的SEM照片(b)
Fig.1
XRD patterns of Li2Sn1-x Si x O3(x=0, 0.05, 0.1) (a) and SEM photograph of LSO (b)
表1 Li2Sn1-x Si x O3(x=0,0.05,0.1)的晶格参数
Table 1
| Samples | Lattice parameters/nm | Lattice volume/nm3 | ||
|---|---|---|---|---|
| Li2SnO3 | a=0.52950 | b=0.91840 | c=1.00320 | V=0.48024 |
| Li2Sn0.95Si0.05O3 | a=0.53001 | b=0.91695 | c=1.00143 | V=0.47923075 |
| Li2Sn0.90Si0.10O3 | a=0.53007 | b=0.91625 | c=1.00157 | V=0.47919176 |
2.2 漫反射光谱
图2
图2
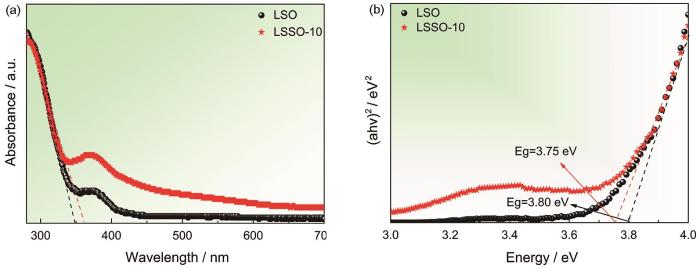
LSO和LSSO-10的UV-vis漫反射谱(a)以及LSO和LSSO-10的(αhv)2与hv的关系(b)
Fig.2
UV-vis diffuse reflectance profiles of LSO and LSSO-10 (a) and relationship between (αhv)2 and hv of LSO and LSSO-10 (b)
2.3 光催化性能
图3
图3
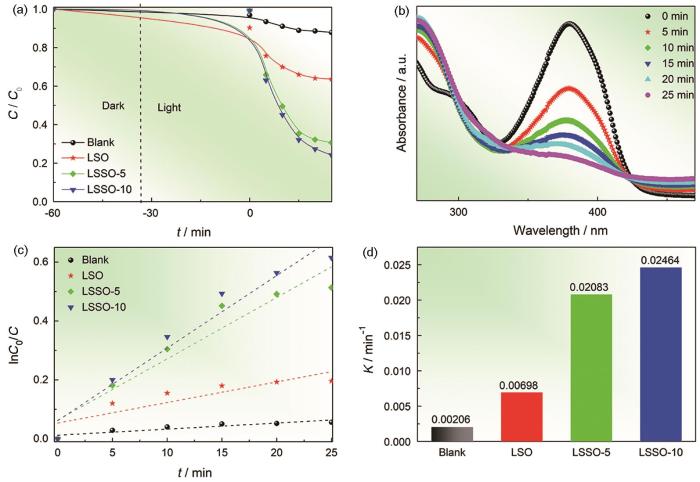
Li2Sn1-x Si x O3(x=0,0.05,0.1)的光催化降解TC图(a)、Li2Sn0.90Si0.10O3 UV下光催化降解TC的紫外吸收光谱(b)、光催化降解TC的一阶动力学线性拟合(c)以及TC光降解拟合的动力学常数(d)
Fig.3
Photocatalytic degradation of TC by Li2Sn1-x SiSiO3(x=0,0.05, 0.1) (a), UV absorption spectrum of Li2Sn0.90Si0.10O3 UV photocatalytic degradation of TC (b), First-order kinetic linear fitting of photocatalytic degradation of TC (c) and Kinetic constant fitting of photodegradation of TC (d)
可用赝一级动力学模型
2.4 自由基的捕获
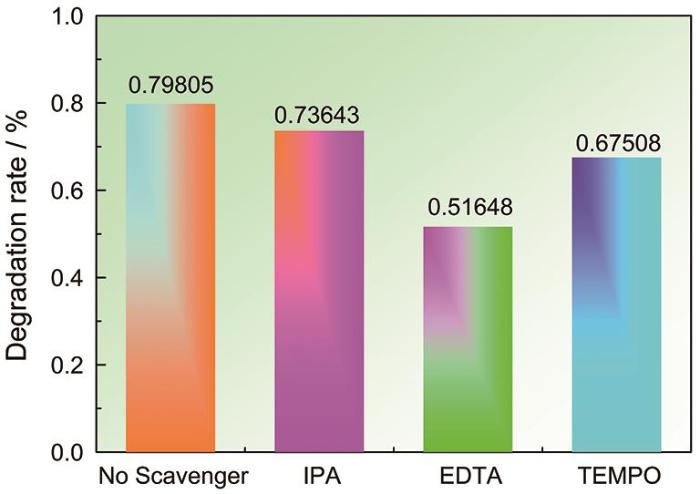
进一步,进行自由基捕获实验以揭示光催化降解机制。如图4所示,分别添加0.1 mmol EDTA,10 ml IPA和0.1 mmol TEMPO后,光催化效率分别为0.73643%、0.51648%和0.67508%。这表明,EDTA和TEMPO的引入抑制了TC溶液的光催化降解效率,其中EDTA的抑制效果显著,而IPA对光催化几乎没有影响,表明催化反应的主要活性物种是h+,
图4
图4
LSSO-10降解TC溶液活性自由基捕获实验
Fig.4
Capture experiment of active free radical in LSSO-10 degradation of TC solution
2.5 理论计算
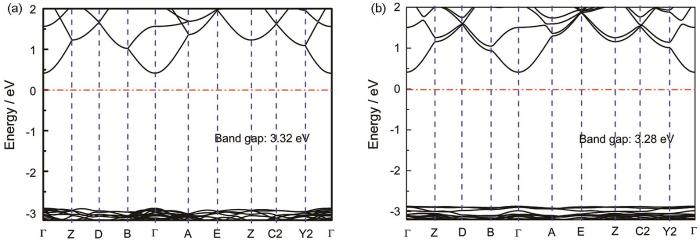
为了更好地理解Si掺杂样品光催化性能的增强,根据第一原理计算Li2SnO3和Li2Sn0.9375Si0.0625O3的电子结构。图5a、b给出了Li2SnO3和Li2Sn0.9375Si0.0625O3的能带图。可以看出,Li2SnO3和Li2Sn0.9375Si0.0625O3均属于直接光学吸收跃迁,价带顶和导带低均处于Γ点。Li2SnO3和Li2Sn0.9375Si0.0625O3的光学吸收带隙分别为3.32 eV和3.28 eV,计算结果略小于从漫反射光谱观察到的结果。Si掺杂使材料的带隙减小,可能是在价带顶形成的杂质能级所致。根据轨道能带图(图6)的计算结果表明,在价带顶形成的杂质能级主要由Si-p,Sn-p,Li-p和O-p轨道占据。
图5
图5
Li2SnO3 (a)和Li2Sn0.9375Si0.0625O3 (b)的能带结构
Fig.5
Energy band structure of Li2SnO3 (a) and Li2Sn0.9375Si0.0625O3 (b)
图6
图6
Li2Sn0.9375Si0.0625O3的轨道能级投影图
Fig.6
Projection diagram of orbital energy levels of Li2Sn0.9375Si0.00625O3
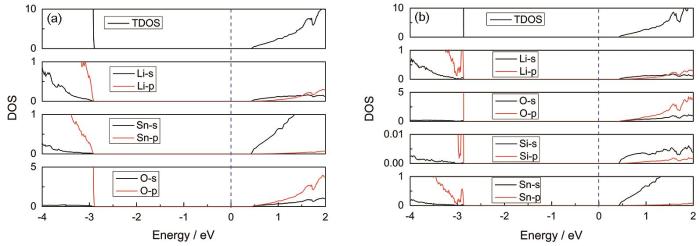
为了进一步理解价带顶形成的杂质能级,计算了Li2SnO3和Li2Sn0.9375Si0.0625O3的电子态密度。从图7a可见,Li2SnO3价带顶主要由O-p轨道构成,而导带底由Sn-s轨道构成。Si掺杂的结果表明,在价带顶形成了新的Si-O,Li-O和Sn-O化学键,从而减小了材料的光学吸收带隙,与能带投影图一致,
图7
图7
Li2SnO3(a)和Li2Sn0.9375Si0.0625O3(b)的电子态密度
Fig.7
Electronic state density of Li2SnO3 (a) and Li2Sn0.9375Si0.0625O3 (b)
2.6 光催化机制
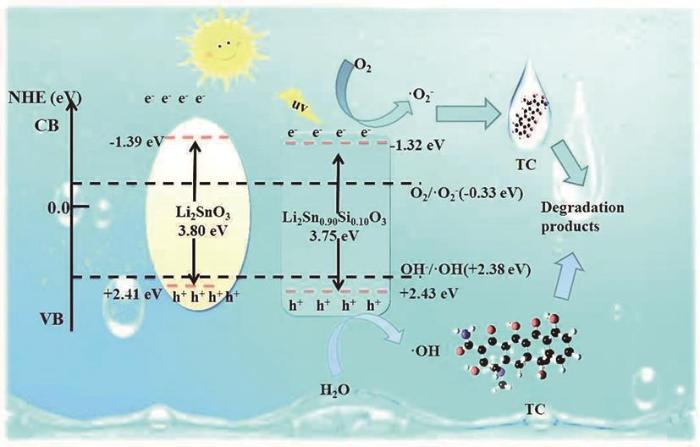
可计算半导体的导带和价带位置。式中χ为半导体的绝对电负性,Eg为禁带宽度,Ee为自由电子的能量(≈4.5 eV)。由此可算出LSO的EVB和ECB分别为2.41 eV和-1.39 eV,而LSSO-10的EVB和ECB分别为2.43 eV和-1.32 eV。
基于上述分析提出了Li2Sn0.90Si0.10O3的光催化机理。涉及到半导体光催化氧化降解污染物机制的活性物种,有·OH,
图8
图8
Li2Sn0.90Si0.10O3光催化剂在紫外光照射下光催化降解TC的机理
Fig.8
Mechanism diagram of photocatalytic degradation of TC by Li2Sn0.90Si0.10O3 photocatalyst under ultraviolet light irradiation
3 结论
用Si掺杂Li2SnO3制备Li2Sn1-x Si x O3(x=0,0.05,0.1),能显著提高其对TC溶液的光催化效率。Li2Sn0.9Si0.1O3样品在25 min内的光催化效率达到最大值(为75.8%),为母体Li2SnO3催化效率的2倍。Si掺杂Li2SnO3在Li2Sn0.9Si0.1O3价带顶形成的杂质能级减小了材料的光学禁带宽度而使光吸收增加,从而提高了材料的光催化效率。在TC的降解中h+起主要作用,而
参考文献
Semiconductor heterojunction photocatalysts: design, construction, and photocatalytic performances
[J].
Aravindakumar. Emerging contaminants in Indian environmental matrices-A review
[J].
Mechanisms for strong adsorption of tetracycline to carbon nanotubes: a comparative study using activated carbon and graphite as adsorbents
[J].
Efficient photocatalytic degradation and adsorption of tetracycline over type-II heterojunctions consisting of ZnO nanorods and K-doped exfoliated g-C3N4 nano-sheets
[J].
The interplay of sulfur doping and surface hydroxyl in band gap engineering: Mesoporous sulfur-doped TiO2 coupled with magnetite as a recyclable, efficient, visible light active photocatalyst for water purification
[J].
Zn4B6O13: Efficient Borate Photocatalyst with Fast Carrier Separation for Photodegradation of Tetracycline
[J].
Phase role of white TiO2 precursor in its reduction to black TiO2
[J].
Highly enhanced visible-light-driven photoelectrochemical performance of ZnO-modified In2S3 nanosheet arrays by atomic layer deposition
[J].
Anionic group self-doping as a promising strategy: band-gap engineering and multi-functional applications of high-performance CO3 2–- doped Bi2O2CO3
[J].
Study on photocatalytic performance of copper doped titanium dioxide
[J].
铜掺杂二氧化钛光催化性能研究
[J].
Li2SnO3 as a cathode material for lithium-ion batteries: defects, lithium ion diffusion and dopants
[J].
SnO2 model electrode cycled in Li-ion battery reveals the formation of Li2SnO3 and Li8SnO6 phases through conversion reactions
[J].
Li2SnO3 branched nano- and microstructures with intense and broadband white-light emission
[J].
Novel high efficiency layered oxide photocatalyst Li2SnO3 for rhodamine B and tetracycline degradation
[J].
Synthesis and luminescence properties of Li2SnO3:Mn4+ red-emitting phosphor for solid-state lighting
[J].
Generalized gradient approximation made simple
[J].
Special points for Brillouin-zone integrations
[J].
KF.S, R. H. Die Kristallstruktur von Li2SnO3
[J].
Highly efficient photocatalytic degradation of amido black 10B dye using polycarbazole-decorated TiO2 nanohybrids
[J].
In situ construction of a MgSn(OH)6 perovskite/SnO2 type-II heterojunction: a highly efficient photocatalyst towards photodegradation of tetracycline
[J].
Facile photochemical synthesis of Au/Pt/g-C3N4 with plasmon-enhanced photocatalytic activity for antibiotic degradation
[J].
Theoretical perspective of photocatalytic properties of single-layer SnS2
[J].
Hydrothermal synthesis and photocatalytic properties of Cu-doped BiVO4 microplates
[J].
Cu掺杂BiVO4微米片的水热合成和光催化性能
[J].
Preparation of ZnTiO3-TiO2 composite photocatalyst and mechanism of photocatalytic degradation of organic pollutants
[J].
ZnTiO3-TiO2复合光催化剂的制备及光催化降解有机污染物机制分析
[J].
In situ construction of the BiOCl/Bi2Ti2O7 heterojunction with enhanced visible-light photocatalytic activity
[J].
Design of visible-light-response core-shell Fe2O3/CuBi2O4 heterojunctions with enhanced photocatalytic activity towards the degradation of tetracycline: Z-scheme photocatalytic mechanism insight
[J].
Photocatalytic reduction performance of palladium-nitrogen co-doped TiO2 films
[J].
钯氮共掺杂TiO2薄膜的光催化还原性能
[J].
Preparation and photocatalytic activity of Bi4Ti3O12/TiO2 heterojunction
[J].
Bi4Ti3O12/TiO2异质结的制备及其光催化性能
[J].
Preparation of CsTi2NbO7@N-TiO2 hybrid core-shell structure and its visible light catalytic activity
[J].
CsTi2NbO7@N-TiO2杂化核壳结构的制备及其可见光催化性能
[J].