1993年R.S.Rouff等[1]首次用电弧放电法制备出碳包覆LaC2的纳米复合材料,发现惰性的碳壳有许多优点[1, 2]:碳材料在酸碱条件下比较稳定,能保护被包覆的金属核不受环境的影响,使其抗氧化能力提高;提高了纳米粒子在极性溶剂中的分散性,阻碍其团聚;提高了材料的导电性能。自此碳包覆金属纳米材料受到了极大的关注,在光学、锂离子电池电极材料、超级电容器、生物医药、催化化学及环境工程等领域得到了应用[3~10]。目前制备碳包覆纳米材料的方法有十余种,除了Ruoff 等采用的电弧放电,还有化学气相沉积法、激光辐照蒸发、溅射和热解法等[11~15]。热解法的特点是,制备装置简单、成本低、一次产物较多、节能。
1 实验方法
1.1 NCCC的制备
先将脱脂棉置于60℃烘箱中干燥至恒重,备用。取适量质量比为10∶1的脱脂棉和五水硫酸铜。将五水硫酸铜溶解在适量的纯水中,加入脱脂棉充分吸附硫酸铜溶液后静置12 h。将吸附了硫酸铜的脱脂棉(Cotton@Cu)置于管式炉(BTF-1200C-4ZL)中,在氮气气氛下以10℃/min的速率升温到390℃并保温1 h,自然冷却到室温,得到NCCC样品。
1.2 商业纳米铜/微米铜(Nano-Cu/Micro-Cu)的预处理
将适量的商业纳米铜和微米铜分别置于马弗炉中,以10℃/min的速率升温到300℃并保温1 h,自然冷却到室温后得到Nano-Cu-air/Micro-Cu-air样品。
1.3 碳包覆纳米铜/微米铜(Nano-Cu/Micro-Cu)用品的制备
将适量的Cotton@Cu和Nano-Cu-air/Micro-Cu-air置于管式炉中,在流量为100 mL/min的氮气氛中以10℃/min的速率升温到390℃并保温1 h,自然冷却到室温后得到Nano-Cu-air-Cotton/Micro-Cu-air-Cotton样品。为了比较,不加Cotton@Cu,只用适量的Nano-Cu-air/Micro-Cu-air,制备出Nano-Cu-air-N2/Micro-Cu-air-N2样品。
1.4 材料性能的表征
用透射电子显微镜(Tecnai G2 F20 S-Twin,FEI)观察用品的微观结构,用X射线衍射分析仪(D8 Advance,Bruker AXS)测试用品的XRD谱,用型号为SDT Q600,TA的热重分析仪进行热分析,用激光显微共聚焦拉曼光谱仪(DXR 2xi,ThermoFisher)测试样品的拉曼光谱。
2 结果和讨论
2.1 NCCC用品的核壳结构
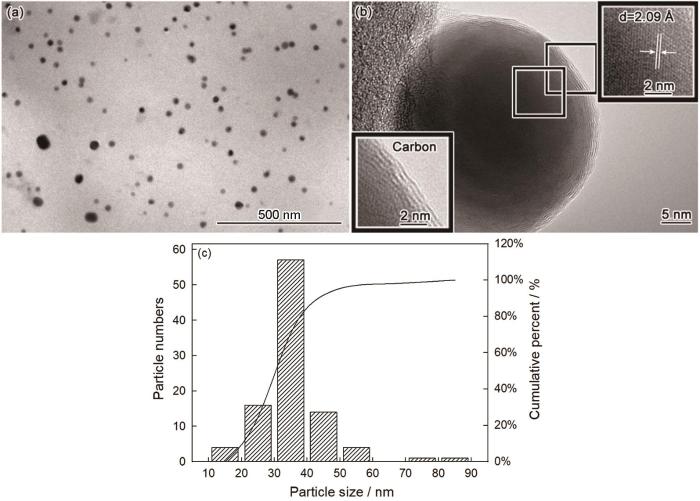
图1
图1
NCCC的TEM照片和NCCC中铜颗粒粒径的统计
Fig.1
TEM images of NCCC (a, b) and particle size of copper particles (c)
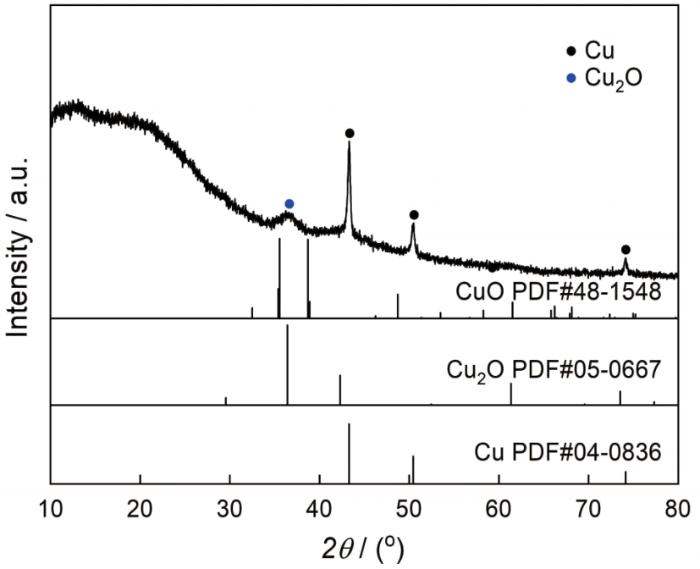
为了确认NCCC的物相组成,测试其XRD谱。从图2可以看出,在390℃碳化后棉纤维吸附的硫酸铜已还原成铜(JCPDS Card No.04-0836)和少量的氧化亚铜(JCPDS Card No.05-0667)。棉纤维在氮气气氛下热降解的过程中产生大量的醛、酮、CO等还原性物质。这些还原性物质将二价铜离子还原为铜单质和少量的氧化亚铜。在XRD谱中除了铜和氧化亚铜的衍射峰外,在10°~30°还出现了两个凸包峰,说明棉纤维在390℃的碳化产物大部分为无定型碳;未出现石墨的特征峰,表明石墨化的碳量极少。根据TEM照片和XRD谱给出的结果,NCCC是典型的碳包覆纳米铜核壳结构的材料。
图2
2.2 碳包覆商业纳米/微米铜的组成
图3
图3
Nano-Cu、Nano-Cu-air、Nano-Cu-air-Cotton和Nano-Cu-air-N2的TEM照片
Fig.3
TEM images of Nano-Cu (a, b), Nano-Cu-air (c, d), Nano-Cu-air-Cotton (e, f) and Nano-Cu-air-N2 (g, h)
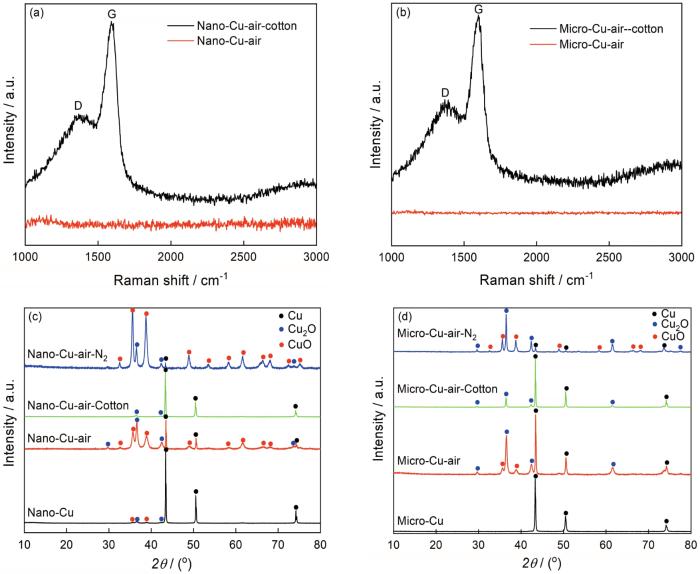
图4
图4
Nano-Cu和Micro-Cu热处理前后的拉曼谱和XRD谱
Fig.4
Raman spectrum and XRD patterns of Nano-Cu and Micro-Cu before and after treatment
图5
图5
Nano-Cu热重分析图
Fig.5
TGA curves of Nano-Cu, (a) tested in N2 atmosphere (10℃/min), (b) tested in air atmosphere (10℃/min)
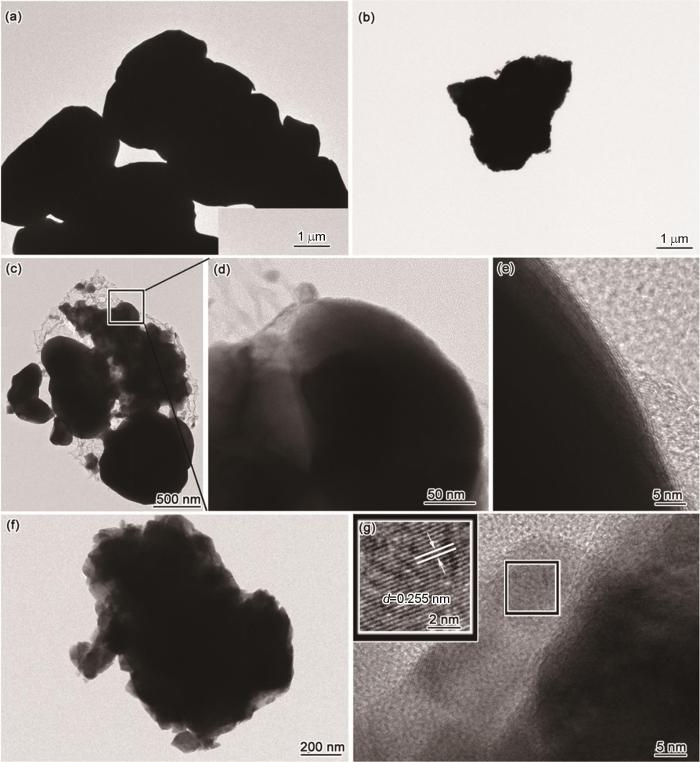
从图3c,d可以看出,在300℃空气预处理后Nano-Cu-air有明显的团聚,有的颗粒已经熔化,金属颗粒表面无壳。从图3e可见,Nano-Cu-air-Cotton呈现团聚状态,金属颗粒熔化,金属颗粒表面产生了不规则的壳,厚度为5~50 nm。从图3f可清晰地看出,Nano-Cu-air-Cotton表面有一层很均匀的壳,厚度约为5 nm。从氮气气氛中对照样Nano-Cu-air-N2的TEM照片可见,金属颗粒表面未形成壳。这表明,棉纤维热解产生的气氛是材料表面形成壳的直接原因。为了证实热解气氛还原后材料表面的壳为碳壳,对Nano-Cu-air-Cotton进行了拉曼分析。从图4a Nano-Cu-air-Cotton的拉曼谱图可以看出,在1376 cm-1和1592 cm-1处出现了特征峰,对应于碳的D峰和G峰。根据IG/ID的比值可衡量碳材料的有序度[18~20]。代表无序结构的D峰比较平缓,代表有序结构的G峰比较尖锐。IG/ID的比值为2.14,表明Nano-Cu-air-Cotton中有一定量的石墨化碳。与Nano-Cu-air的拉曼谱对比表明,经过热解碳源还原后得到Nano-Cu-air-Cotton,其表面确实有碳壳。将图4c给出的物相组成与Nano-Cu-air-N2相比可见,Nano-Cu-air-Cotton在棉纤维的热解气氛中发生了还原(表1)。从XRD物相组成可以看出,CuO已还原为氧化亚铜或铜单质。这也进一步证实了棉纤维的热解气氛的还原作用,解释了NCCC中单质铜的形成。
表1 商业纳米铜处理前后的物相组成
Table 1
| Sample name | Phase composition | ||
|---|---|---|---|
| Cu | Cu2O | CuO | |
| Nano-Cu | √ | √ | √ |
| Nano-Cu-air | √ | √ | √ |
| Nano-Cu-air- N2 | √ | √ | √ |
| Nano-Cu-air- Cotton | √ | √ | × |
为了探究相同实验条件下棉纤维热解气氛对微米铜的影响,对微米铜样品进行相同的预处理。从图6a可以看出,Micro-Cu为不规则颗粒,出现了明显的团聚,颗粒尺寸为8 μm×3 μm;图6b给出了在300℃空气中预处理样品的的TEM照片,可见处理前后样品表面都没有壳层。经过棉纤维热解气氛还原后,微米铜颗粒周围包裹了壳层(图6c)。将图6c的照片放大(图6d, e),可见颗粒表面覆盖了均匀的壳层。从氮气气氛对照样Micro-Cu-air-N2的TEM照片可见,颗粒的周围没有壳层。这个结论和纳米铜的验证结果一致,进一步证实棉纤维热解气氛是金属颗粒壳层形成的决定性因素。为了探究颗粒表面的壳层是否为碳壳,对其进行了拉曼分析。在图4b给出的Micro-Cu-air-Cotton拉曼谱中1365 cm-1和1598 cm-1处分别出现了对应于碳的D峰和G峰。IG/ID的比值为2.00,结合Micro-Cu-air-Cotton的TEM照片,表明Micro-Cu-air-Cotton中有一定量石墨化的无定型碳。与Micro-Cu-air的拉曼谱对比表明,Micro-Cu-air-Cotton表面的壳确实为碳壳。XRD谱给出的物相分析结果(图4d和表2)表明,Micro-Cu经空气热处理后单质铜氧化为氧化亚铜和氧化铜,经棉纤维热分解气氛热处理后得到Micro-Cu-air-Cotton,氧化铜被还原。而对照样品Micro-Cu-air-N2中仍然有氧化铜,证实了棉纤维热分解气氛的还原作用。
图6
图6
Micro-Cu、Micro-Cu-air、Micro-Cu-air-Cotton和Micro-Cu-air-N2的TEM照片
Fig.6
TEM images of Micro-Cu (a), Micro-Cu-air (b), Micro-Cu-air-Cotton (c, d, e), and Micro-Cu-air-N2 (f, g)
表2 商业微米铜处理前后的物相组成
Table 2
| Sample name | Phase composition | ||
|---|---|---|---|
| Cu | Cu2O | CuO | |
| Micro-Cu | √ | × | × |
| Micro-Cu-air | √ | √ | √ |
| Micro-Cu-air- N2 | √ | √ | √ |
| Micro-Cu-air- Cotton | √ | √ | × |
上述原位热解实验结果表明,棉纤维热解气氛为商业纳米铜和微米铜核壳结构的形成提供了碳源,热解气氛具有一定的还原作用。这就给出了NCCC核壳结构中碳壳、单质铜和氧化亚铜的形成机理。
2.3 核壳结构纳米铜的稳定性
图7
图7
碳包覆纳米铜在不同环境放置不同时间后的XRD谱
Fig.7
XRD patterns of carbon-capsulated nano-copper particles stored in different environment for different time (a) NCCC stored in different environment for different time; (b) Nano-Cu stored for one day and 120 d; (c) Nano-Cu-air-Cotton stored for 1 d and 120 d
3 结论
(1) 用一步热解法原位制备的核壳结构的纳米铜碳材料(NCCC),单质铜纳米颗粒均匀地镶嵌在碳基体中,大部分粒径为15~50 nm。以浸泡了硫酸铜的棉纤维为热解碳源、以商业纳米铜和微米铜为铜源可原位制备碳包覆纳米/微米铜。
(2) 用一步热解法制备碳包覆金属颗粒材料,核壳结构中的碳壳使材料的稳定性和抗氧化性提高。
参考文献
Single-crystal metals encapsulated in carbon nanoparticles
[J].Single-domain microcrystals of LaC(2) encapsulated within nanoscale polyhedral carbon particles have been synthesized in a carbon arc. Typical particle sizes are on the order of 20 to 40 nanometers. The stoichiometry and phase of the La-containing crystals have been assigned from characteristic lattice spacings observed by high-resolution transmission electron microscopy and energy dispersive spectroscopy (EDS). EDS spectra show that La and C are the only elements present. Characteristic interatomic distances of 3.39 and 2.78 angstroms identify the compound inside the nanoparticle cavities as alpha-LaC(2), the phase of LaC(2) that is stable at room temperature. Bulk alpha-LaC(2) is metallic and hydrolytic. Observation of crystals of pure encapsulated alpha-LaC(2) that were exposed to air for several days before analysis indicates that the LaC(2) is protected from degradation bythe carbon polyhedral shells of the nanoparticles. A high percentage of the carbon nanoparticles have encapsulated LaC(2) single crystals. These carbon-coated metal crystals form a new class of materials that can be protected in their pure or carbide forms and may have interesting and useful properties.
Thermal synthesis of Cu@carbon spherical core-shell structures from carbonaceous matrices containing embedded copper particles
[J].
Porous Fe3O4/Carbon core/shell nanorods: synthesis and electromagnetic properties
[J].
Synthesis, growth mechanism and thermal stability of copper nanoparticles encapsulated by multi-layer graphene
[J].
The preparation of carbon-encapsulated Fe/Co nanoparticles and their novel applications as bifunctional catalysts to promote the redox reaction for p-nitrophenol
[J].
Design, synthesis and applications of core-shell, hollow core, and nanorattle multifunctional nanostructures
[J].With the evolution of nanoscience and nanotechnology, studies have been focused on manipulating nanoparticle properties through the control of their size, composition, and morphology. As nanomaterial research has progressed, the foremost focus has gradually shifted from synthesis, morphology control, and characterization of properties to the investigation of function and the utility of integrating these materials and chemical sciences with the physical, biological, and medical fields, which therefore necessitates the development of novel materials that are capable of performing multiple tasks and functions. The construction of multifunctional nanomaterials that integrate two or more functions into a single geometry has been achieved through the surface-coating technique, which created a new class of substances designated as core-shell nanoparticles. Core-shell materials have growing and expanding applications due to the multifunctionality that is achieved through the formation of multiple shells as well as the manipulation of core/shell materials. Moreover, core removal from core-shell-based structures offers excellent opportunities to construct multifunctional hollow core architectures that possess huge storage capacities, low densities, and tunable optical properties. Furthermore, the fabrication of nanomaterials that have the combined properties of a core-shell structure with that of a hollow one has resulted in the creation of a new and important class of substances, known as the rattle core-shell nanoparticles, or nanorattles. The design strategies of these new multifunctional nanostructures (core-shell, hollow core, and nanorattle) are discussed in the first part of this review. In the second part, different synthesis and fabrication approaches for multifunctional core-shell, hollow core-shell and rattle core-shell architectures are highlighted. Finally, in the last part of the article, the versatile and diverse applications of these nanoarchitectures in catalysis, energy storage, sensing, and biomedicine are presented.
Core-shell materials for advanced batteries
[J].Nowadays, materials with a core-shell structure have been widely explored for applications in advanced batteries owing to their superb properties. Core-shell structures based on the electrode type, including anodes and cathodes, and the material compositions of the cores and shells have been summarized. In this review, we focus on core-shell materials for applications in advanced batteries such as LIBs, LSBs and SIBs. Firstly, a novel concept of aggregates of spherical core-shell architectures and their aggregates, linear core-shell architectures and their aggregates, sheet-like core-shell architectures and their aggregates and special core-shell architectures and their aggregates are involved. Secondly, the main material compositions of carbon/Si-based, carbon/metal-based, metal-based materials and organic-based composites are introduced along with the synthesis and electro-chemical performances of core-shell nanostructured materials. Finally, the emerging challenges and prospects of core-shell materials are briefly discussed.
Enhanced solar thermal conversion performance of plasmonic gold dimer nanofluids
[J].
Modeling the solar absorption performance of Copper@Carbon core-shell nanoparticles
[J].
Preparation and properties of carbon coated manganese dioxide electrode materials
[J].Manganese dioxide powders were firstly prepared via electric pulse assisted redox method with KMnO4 and MnSO4 as raw material, then MnO2/C composite materials coated with different amounts of carbon were fabricated via liquid phase sintering with glucose as a carbon source. The effect of amount of coated carbon on the morphology, structure and electrochemical properties of the MnO2/C materials were investigated. Results show that the coated carbon could induce the transformation of crystallographic structure of MnO2 from γ-type into α-type. Under heating conditions glucose decomposed and coated on the surface of MnO2 particles, which could inhibit the grain growth and thus refine grains. When the preparation with the process parameters: glucose concentration was 1.5 g/L and the current density was 2 A·g-1, the prepared MnO2/C material presented the specific capacitance of MnO2 of 722.2 F·g-1, in other words, the carbon coating could increase the specific capacitance by 80%, in comparison with that of the blank ones. Furthermore, after 4000 charge-discharge cycles, the capacitance retention rate could still maintain 74.72%, displayed good electrochemical performance and cycling performance.
碳包覆改性二氧化锰电极材料的制备和性能
[J].
Synthesis of carbon nanocapsules containing Fe, Ni or Co by arc discharge in aqueous solution
[J].
Li ion battery materials with core–shell nanostructures
[J].
Cu and Cu-based nanoparticles: synthesis and applications in catalysis
[J].The applications of copper (Cu) and Cu-based nanoparticles, which are based on the earth-abundant and inexpensive copper metal, have generated a great deal of interest in recent years, especially in the field of catalysis. The possible modification of the chemical and physical properties of these nanoparticles using different synthetic strategies and conditions and/or via postsynthetic chemical treatments has been largely responsible for the rapid growth of interest in these nanomaterials and their applications in catalysis. In addition, the design and development of novel support and/or multimetallic systems (e.g., alloys, etc.) has also made significant contributions to the field. In this comprehensive review, we report different synthetic approaches to Cu and Cu-based nanoparticles (metallic copper, copper oxides, and hybrid copper nanostructures) and copper nanoparticles immobilized into or supported on various support materials (SiO2, magnetic support materials, etc.), along with their applications in catalysis. The synthesis part discusses numerous preparative protocols for Cu and Cu-based nanoparticles, whereas the application sections describe their utility as catalysts, including electrocatalysis, photocatalysis, and gas-phase catalysis. We believe this critical appraisal will provide necessary background information to further advance the applications of Cu-based nanostructured materials in catalysis.
Carbon nanocages with N-doped carbon inner shell and Co/N-doped carbon outer shell as electromagnetic wave absorption materials
[J].
Preparation, formation mechanism and optical properties of C/Cu shell/core nanostructures
[J].
Cu/C核/壳纳米结构的气相合成、形成机理及其光学性能研究
[J].
Preparation and magnetic behavior of carbon-encapsulated cobalt and nickel nanoparticles from starch
[J].
Inspired by efficient cellulose-dissolving system: facile one-pot synthesis of biomass-based hydrothermal magnetic carbonaceous materials
[J].
Photochemical chlorination of graphene
[J].We report the covalent functionalization of graphene by photochemical chlorination. The gas-phase photochlorination of graphene, followed by the structural transformation of the C-C bonds from sp(2) to sp(3) configuration, could remove the conducting π-bands and open up a band gap in graphene. X-ray photoelectron spectroscopy revealed that chlorine is grafted to the basal plane of graphene, with about 8 atom % chlorine coverage. Raman spectroscopy, atomic force microscopy, and transmission electron microscopy all indicated that the photochlorinated graphene is homogeneous and nondestructive. The resistance increases over 4 orders of magnitude and a band gap appears upon photochlorination, confirmed by electrical measurements. Moreover, localized photochlorination of graphene can facilitate chemical patterning, which may offer a feasible approach to the realization of all-graphene circuits.
Strong charge-transfer doping of 1 to 10 layer graphene by NO2
[J].We use resonance Raman and optical reflection contrast methods to study charge transfer in 1-10 layer (1L-10L) thick graphene samples on which NO(2) has adsorbed. Electrons transfer from the graphene to NO(2), leaving the graphene layers doped with mobile delocalized holes. Doping follows a Langmuir-type isotherm as a function of NO(2) pressure. Raman and optical contrast spectra provide independent, self-consistent measures of the hole density and distribution as a function of the number of layers (N). At high doping, as the Fermi level shift E(F) reaches half the laser photon energy, a resonance in the graphene G mode Raman intensity is observed. We observe a decrease of graphene optical absorption in the near-IR that is due to hole-doping. Highly doped graphene is more optically transparent and much more electrically conductive than intrinsic graphene. In thicker samples, holes are effectively confined near the surface, and in these samples, a small band gap opens near the surface. We discuss the properties and versatility of these highly charge-transfer-doped, few-layer-thick graphene samples as a new class of electronic materials.