过渡金属氧化物的丰度大且具有较高的理论比容量(600~1200 mAh·g-1) [8],是极具应用价值的负极材料之一。Co3O4的理论比容量较高(890 mAh·g-1)[9,10],能提高锂离子电池的容量,但是在脱嵌锂过程中发生的体积膨胀使活性物质脱落、容量衰减快和循环性能降低[11,12]。控制反应条件使Co3O4纳米化,可提供更多的反应位点,减小锂离子扩散路径[13]。同时,与导电材料(如:Co3O4@C[14,15],Co3O4@Graphene[16,17],Co3O4@ CNTs[18,19])复合可提高Co3O4的电子电导率,缓解氧化物负极材料在循环过程中的体积膨胀。在传统的电池制备工艺中使用粘结剂和导电剂与活性材料混合可使材料均一化成为一个稳定的系统,但是材料在循环中的体积膨胀破坏了体系的稳定性,使其分层、脱落和容量下降[20]。不使用粘结剂和导电剂有助于保持体系的一致性,提高电池可逆容量。本文使用简单的一步水热反应将Co3O4纳米颗粒负载于碳纳米管薄膜上制备三维Co3O4@CNTs复合材料薄膜,并研究其储锂性能。
1 实验方法
1.1 材料的制备
将摩尔比为1:1:1的CoSO4·7H2O(AR,麦克林)、5-磺基水杨酸(AR)和戊二酸(AR)溶于50 mL去离子水中,用NaOH(AR,西陇化工)将溶液的pH值调节至7.0。在溶液中加入大小为2 cm×2 cm、厚度6 μm的碳纳米管薄膜,然后转移至聚四氟乙烯内衬的反应釜中,在140℃水热反应24 h制得Co3O4@CNTs薄膜复合材料。使用5-磺基水杨酸和戊二酸作为氧化剂将Co2+部分氧化成Co3+,CNFs提供成核位点使Co3O4能够负载于其上形成Co3O4@CNTs复合物。Co3O4与CNTs薄膜的质量比约为85:15。将制得的薄膜复合材料洗涤和干燥后裁成直径为14 mm的圆片。为了对比,在同等条件下合成Co3O4纳米粉体,并及其与导电剂SP、粘结剂PVDF按质量比75:15:10混合均匀,涂覆在铜箔后进行干燥和冲片,然后在真空手套箱中组装电池。实验流程如图1所示。
图1
1.2 结构和性能表征
用D8 Advance X射线衍射(XRD)仪进行物相分析(扫描条件:Cu Kα辐射,λ=0.15406 nm,扫描范围:2θ=10°~80°,步长为0.02°,扫描速率为2°/min);用ZEISS SIGMA 300型扫描电子显微镜(SEM)观测产物的颗粒形貌;用 Thermo SCIENTIFIC ESCALAB 250Xi型X射线光电子能谱(XPS)进行元素价态分析。使用新威电池测试系统在室温下测试电池的循环性能和倍率性能,用Ivium-n-Stat型电化学工作站进行CV性能测试,测试范围0.01~3 V,扫描速率为0.1 mV·s-1。使用上海辰华电化学工作站进行交流阻抗测试(EIS),频率范围0.01~100 kHz,振幅为0.01 V·s-1。
2 结果和讨论
2.1 Co3O4和Co3O4@CNTs的晶体结构和形貌
图2
图2
Co3O4和Co3O4@CNTs的X射线衍射图谱
Fig.2
XRD pattern of Co3O4 and Co3O4@CNTs film samples
图3
图3
Co3O4粉体和Co3O4@CNTs薄膜的扫描电镜照片
Fig.3
SEM images of Co3O4 power (a, b) and Co3O4@CNTs film (c, d)
图4
图4
Co3O4的X射线光电子能谱图
Fig.4
XPS patterns whole element (a) and high resolution of Co 2p (b) of Co3O4
2.2 Co3O4粉体和Co3O4@CNTs的电化学性能
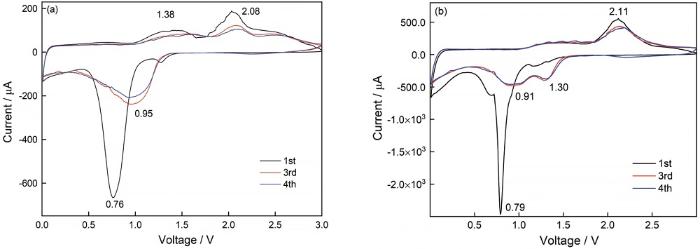
图5给出了Co3O4和Co3O4@CNTs电极材料在0.01~3 V区间的循环伏安曲线。在首圈阴极扫描中,两种材料分别在0.76 V和0.79 V处存在极强的阴极峰,表明其负极材料循环过程中的不可逆容量,这是由于负极材料表面SEI膜的形成和副反应的产生。这是过渡金属氧化物负极材料的初始不可逆容量大,库伦效率低的主要原因[25,26]。在图5a中,阳极扫描曲线出现了两个明显的氧化峰(1.38 V和2.08 V),对应Co→Co2+/Co3+。在随后的扫描曲线中氧化峰峰位逐渐往高电压漂移,说明材料发生了极化,对应阴极峰仅出现在0.95V处,说明单纯合成的Co3O4粉体由于团聚到一起,阻碍了锂离子的嵌入通道。从图5b中可以看出,Co3O4@CNTs电极材料在0.91 V和1.30 V出现两个还原峰,对应放电过程中的Co3+→Co2+/Co2+→Co转变,相应的氧化峰出现在1.36 V和2.11 V对应金属Co向Co3O4的转变[27]。后续的CV曲线重合性高,还原电位更小,电流密度更高,说明该材料比纯Co3O4电极具有更强的储锂能力和更优异的循环稳定性。
图5
图5
Co3O4(a)和Co3O4@CNTs(b)薄膜电极的循环伏安曲线
Fig. 5
CVs of Co3O4 (a) and Co3O4@CNTs (b) electrode scanned between 0.01~3 V (vs. Li/Li+) at a scan rate of 0.1 mV·s-1
图6a和b分别给出了Co3O4和Co3O4@CNTs在50 mA·g-1电流密度下的充放电曲线。在图6a中,首次循环的放电平台在1V附近,与CV曲线相对应,放电比容量达到1117.8 mAh·g-1。在图6b中,Co3O4@CNTs电极也在1V处存在较长的放电平台,其容量达到2313.2 mAh·g-1。两种材料的初始比容量均远高于Co3O4的理论比容量,因为脱嵌锂过程引起副反应发生,SEI膜的形成也占据消耗了大量的Li+。Co3O4@CNTs膜材料首次库伦效率为68.2%,低于Co3O4材料的库伦效率72.2%。其主要原因是,碳纳米管薄膜在材料放电过程中容纳了部分金属锂,未完全释放出来[28]。在2nd和3rd循环中库伦效率上升至95%,且充放电曲线重合度极高,表明薄膜复合材料具有更好的循环稳定性。
图6
图6
电流密度为50 mA·g-1条件下Co3O4和Co3O4@CNTs薄膜电极材料的充放电曲线
Fig.6
Charge-Discharge curves of Co3O4 (a) and Co3O4@CNTs (b) at 50 mA·g-1 current density
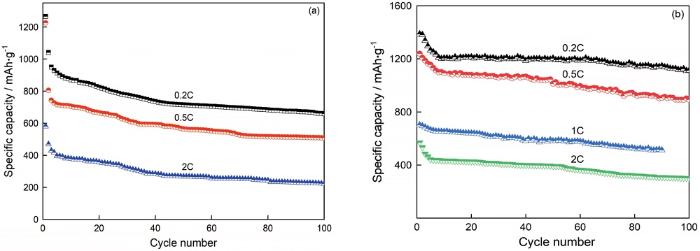
图7给出了Co3O4和Co3O4@CNTs薄膜电极材料在不同倍率下的循环测试。在图7a中,Co3O4电极在前30次循环中容量衰减较快,之后衰减趋于平缓。在0.2C、0.5C、2C电流下100次循环后放电比容量分别为659.3 mAh·g-1、508.8 mAh·g-1和223.3 mAh·g-1,容量保持率为52%、44%、38.5%。图7b中,Co3O4@CNTs电极在0.2C、0.5C、1C、2C电流下表现出优于Co3O4粉体的电化学循环性能和容量保持率,约10次循环后容量基本达到平衡状态,100次循环后放电比容量分别达到1128.9 mAh·g-1、925.1 mAh·g-1、527.8 mAh·g-1(90次)、312.1 mAh·g-1,对应的容量保持率为66%、66.1%、53.8%、47.5%。
图7
图7
Co3O4和Co3O4@CNTs薄膜电极不同倍率下的循环性能
Fig.7
Cycle performance for Co3O4 (a) and Co3O4@CNTs (b) electrodes at different rate
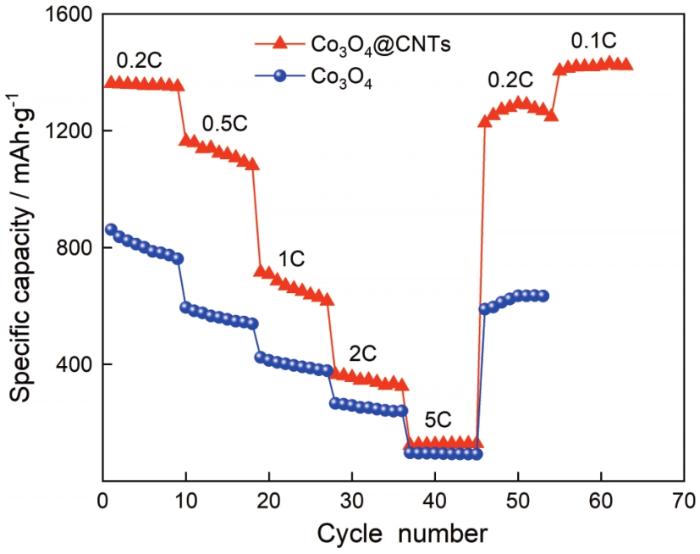
为了进一步探究Co3O4和Co3O4@CNTs电极倍率性能,将两种材料置于不同电流下进行测试,如图8所示。可以看出,Co3O4@CNTs电极在0.2C、0.5C、1C、2C、5C电流下循环,其容量分别达到1356.5 mAh·g-1、1124.7 mAh·g-1、663.4 mAh·g-1、344.3 mAh·g-1、126.3 mAh·g-1;经过5C大倍率充放电后,在0.2C小电流下其放电比容量恢复1267 mAh·g-1,为初始值的91.8%;以0.1C低电流放电时容量达到1419.4 mAh·g-1,而Co3O4电极其容量仅为804 mAh·g-1、562.7 mAh·g-1、397.8 mAh·g-1、251.2 mAh·g-1、94.3 mAh·g-1、619.8 mAh·g-1,0.2C时可逆容量仅恢复到初始值的76.2%。这表明,Co3O4@CNTs电极具有优异的倍率性能,能在大电流放电过程中维持结构稳定。Co3O4@CNTs复合材料具有优异的电化学性能得益于Co3O4和CNTs协同作用,表面粗糙的Co3O4粒子能为Li+离子提供更多的反应活性位点,缩短Li+扩散路径。同时,碳纳米管具有优良的导电能力,Co3O4粒子附着于CNFs上可提高复合材料的导电性能。CNFs具有良好的机械性能,能在体积膨胀过程中稳定材料,避免粒子之间因团聚引发粉化和脱落。
图8
图8
Co3O4和Co3O4@CNTs薄膜电极不同倍率下的循环性能
Fig.8
Rate capability of Co3O4 and Co3O4@CNTs electrodes
图9给出了两种电极经历30次循环后进行测试后的Nyquist图,可印证Co3O4@CNTs复合材料降低材料内部电阻的性能。由图9可见,在中-高频区域出现的半圆对应锂离子在电极表面的扩散阻抗和电荷转移阻抗(Rct),在低频区出现的斜线代表锂离子在电解液中的扩散阻抗(W)。两种电极使用相同的电解质,所以高频区的电解质溶液电阻(Rs)非常接近,Co3O4@CNTs电极的Rct比Co3O4电极Rct低近200 Ω·cm2,其Rct与文献[29]报道的材料相近,说明电子在CNFs与Co3O4之间传导更快。其原因是,Co3O4粉体通过碳纳米管薄膜的分散利于活性材料与电解液的浸润,增大活性材料与电解液之间的接触面积,有利于减小锂离子迁移阻抗,且碳纳米管薄膜有利于电极表面的电子转移,进而使活性材料的电化学活性提高。
图9
图9
Co3O4和Co3O4@CNTs 薄膜电极的Nyquist图
Fig.9
Nyquist plots of Co3O4 and Co3O4@CNTs electrodes
图10
图10
Co3O4@CNTs薄膜电极循环100次和酸化处理CNTs薄膜的SEM图
Fig.10
SEM images of Co3O4@CNTs after 100 cycles (a) and after acidification (b)
3 结论
用简单一步水热法可制备出Co3O4@CNTs薄膜复合材料,直接用作锂离子电池的负电极。Co3O4纳米粒子能与碳纳米管薄膜复合,使其具有高比容量、高循环性能稳定和较高的倍率性能。在Co3O4@CNTs薄膜复合材料中碳纳米管薄膜形成三维空间导电网络使其电子传导特性提高。碳纳米管薄膜具有优异的机械性能,可在电化学循环过程中稳定材料的结构。Co3O4纳米粒子在碳纳米管薄膜上的高度分散和膜间隙有利于电解液的充分浸润,增大活性材料与电解液之间的接触面积,从而减小锂离子迁移阻抗、改善负极材料在电化学循环过程中的导电率低和降低体积膨胀的影响。
参考文献
Development status and research progress of power battery for pure electric vehicles
[J].
纯电动车用锂离子电池发展现状与研究进展
[J].
A novel SnO2@BNNSs@C composite nano-structure and its electrochemical energy storage characteristics
[J].
一种新型SnO2@BNNSs@C纳米复合结构及其电化学储能特性
[J].
An experimental study on overcharge behaviors of lithium-ion power battery with LiNi0.6Co0.2Mn0.2-O2 cathode
[J].
三元锂离子动力电池过充行为特性实验研究
[J].
Electrochemical deposition of porous Co3O4 nanostructured thin film for lithium-ion battery
[J].
Improvement of electrochemical performance of LiNi0.8Co0.1Mn0.1O2 cathode material by graphene nanosheets modification
[J].
LiFePO4-Coated Li1.2Mn0.54Ni0.13Co0.13-O2 as cathode materials with high coulombic efficiency and improved cyclability for Li-ion batteries
[J].
LiFePO4包覆的Li1.2Mn0.54Ni0.13Co0.13-O2锂离子电池正极材料: 增强的库伦效率和循环性能
[J].
Improving the electrochemical performance and structural stability of the LiNi0.8Co0.15Al0.05O2 cathode material at high-voltage charging through Ti substitution
[J].
Hydrothermal for synthesis of CoO nanoparticles/graphene composite as li-ion battery anodes
[J].
水热法合成氧化亚钴纳米粒子/石墨烯复合材料及其储锂性能研究
[J].
Co3O4 as anode material for thin film micro-batteries prepared by remote plasma atomic layer deposition
[J].
Grass-like Co3O4 nanowire arrays anode with high rate capability and excellent cycling stability for lithium-ion batteries
[J].
Unique porous yolk-shell structured Co3O4 anode for high performance lithium ion batteries
[J].
Porous polyhedral and fusiform Co3O4 anode materials for high-performance lithium-ion batteries
[J].
Synthesis of nanoparticles-assembled Co3O4 microspheres as anodes for Li-ion batteries by spray pyrolysis of CoCl2 solution
[J].
Adsorption-based synthesis of Co3O4/C composite anode for high performance lithium-ion batteries
[J].
Nanosheets-in-nanotube Co3O4-carbon array design enables stable Li-ion storage
[J].
Hydrothermal preparation of Co3O4/graphene composite as anode material for lithium-ion batteries
[J].
Co3O4-graphene nanoflowers as anode for advanced lithium ion batteries with enhanced rate capability
[J].
Electrochemical properties of CNTs/Co3O4 blended-anode for rechargeable lithium batteries
[J].
Hollow Co-Co3O4@CNTs derived from ZIF-67 for lithium ion batteries
[J].
Causes of binder damage in porous battery electrodes and strategies to prevent it
[J].
Graphene anchored with Co3O4 nanoparticles as anode of lithium ion batteries with enhanced reversible capacity and cyclic performance
[J].We report a facile strategy to synthesize the nanocomposite of Co(3)O(4) nanoparticles anchored on conducting graphene as an advanced anode material for high-performance lithium-ion batteries. The Co(3)O(4) nanoparticles obtained are 10-30 nm in size and homogeneously anchor on graphene sheets as spacers to keep the neighboring sheets separated. This Co(3)O(4)/graphene nanocomposite displays superior Li-battery performance with large reversible capacity, excellent cyclic performance, and good rate capability, highlighting the importance of the anchoring of nanoparticles on graphene sheets for maximum utilization of electrochemically active Co(3)O(4) nanoparticles and graphene for energy storage applications in high-performance lithium-ion batteries.
Enhanced electrochemical performance of flower-like Co3O4 as an anode material for high performance lithium-ion batteries
[J].
CoO-Co3O4 heterostructure nanoribbon/RGO sandwich-like composites as anode materials for high performance lithium-ion batteries
[J].
Co3O4 nanocrystalline-assembled mesoporous hollow polyhedron nanocage-in-nanocage as improved performance anode for lithium-ion batteries
[J].
Chemical reduction-induced oxygen deficiency in Co3O4 nanocubes as advanced anodes for lithium ion batteries
[J].
Performance of lithium-ion capacitors using pre-lithiated multi-walled carbon nanotube composite anode
[J].
预嵌锂多壁碳纳米管的性能
[J].
Facial preparation of N-doped carbon foam supporting Co3O4 nanorod arrays as free-standing lithium-ion batteries’s anode
[J].
Enhanced lithium storage in Co3O4/carbon anode for Li-ion batteries
[J].
High performance porous anode based on template-free synthesis of Co3O4 nanowires for lithium-ion batteries
[J].