本文以ASA为原料制备CDs,再用物理共混非溶剂诱导相分离技术(NIPs)制备CDs/PSF共混纳米复合膜,研究CDs的引入对分离膜性能的影响。
1 实验方法
1.1 实验用材料
4-氨基水杨酸(ASA,99.7%,质量分数)、聚乙二醇-400(PEG-400,99.7%,质量分数)、N, N'-二甲基乙酰胺(DMAc,99.7%,质量分数)、无水乙醇(99.7%,质量分数)、聚砜(PSF S6010)以及透析袋(截留分子量为1.0 kDa)。
1.2 CDs和分离膜的制备
采用非溶剂诱导相分离法制备PSF/CDs复合膜。将PSF(18%,质量分数)、PEG(9%,质量分数)以及一定质量分数的CDs分散在DMAc溶剂中,在80℃匀速搅拌约10 h后静置24 h以去除气泡。将适量的铸膜液置于干净玻璃板上,用150 μm刮刀匀速刮涂并迅速将玻璃板浸入室温水凝固浴中,约2 min后成膜。将生成的分离膜取出并置入清水中,每隔6 h换一次水以除去残留的DMAc和添加剂。按加入CDs的质量分数(%),将分离膜标记为M0、M0.5、M1、M2、M3和M5。
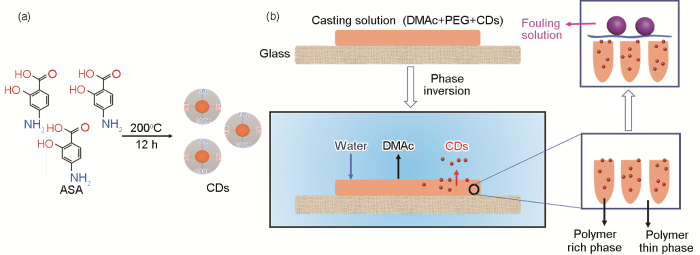
CDs的制备原理和分离膜改性的简单流程,如图1所示。
图1
图1
CDs的合成和相转化成膜过程分离膜内部变化示意图
Fig.1
Schematic diagram of the preparation processes of CDs (a) and the particle motion during the NIPs (b)
1.3 CDs和分离膜的表征
用傅里叶变换红外光谱仪和X射线光电子能谱仪表征CDs和分离膜的化学组成。用透射电子显微镜观察CDs的形貌特征;用扫描电子显微镜观察分离膜的表面形貌和断面形貌;用扫描探针显微镜用来检测分离膜的表面粗糙度。用接触角(CA)分析仪测量分离膜的亲水性。用干湿质量法计算分离膜的孔隙率(%)
其中,m1和m2分别为被水润湿的湿膜状态和未被水润湿的干膜状态时分离膜的质量;ρ1为纯水的密度(0.998 g/cm3);ρ2为PSF的密度(1.240 g/cm3)。
在室温测试分离膜的过滤性能。将完整度较好的分离膜裁切成直径约2.5 cm的圆片,将其固定在测试装置上并在0.2 MPa压力下向测试仪器内注入纯水,压实30 min。待纯水通过速率稳定后将压力降至0.1 MPa。记录单位时间通过膜的纯水的质量并将其转换为体积。测试3个不同配比的分离膜样品,取其结果的平均值。纯水通量(L·m-2·h-1)[16]为
其中,V(L)为在测试时间(t, h)内通过固定面积(S=14.38×10-4 m2)分离膜的水的体积。
采用截留牛血红蛋白分子(BSA)的方法表征分离膜的分离性能。以BSA溶液(生理盐水中浓度为0.8 g/L)替代纯水作为测试液,重复上述测试通量的操作测得BSA溶液通量FBSA;收集滤液以检测滤液中残留的BSA分子含量。将测试后的分离膜置于生理盐水中多次震荡清洗去除污染,重新测量分离膜的纯水通量FW2。根据通量回复率(FRR)、总污染率(Rt)、可逆污染率(Rr)和不可逆污染率(Rir)
BSA分子截留率(RBSA, %):
评估分离膜的防污性能
用紫外可见分光光度计测得280 nm波长处的Ct (g/L)和C0(g/L),Ct(g/L)和C0(g/L)分别为滤液和原BSA溶液中的BSA分子浓度。
2 结果和分析
2.1 CDs的表征
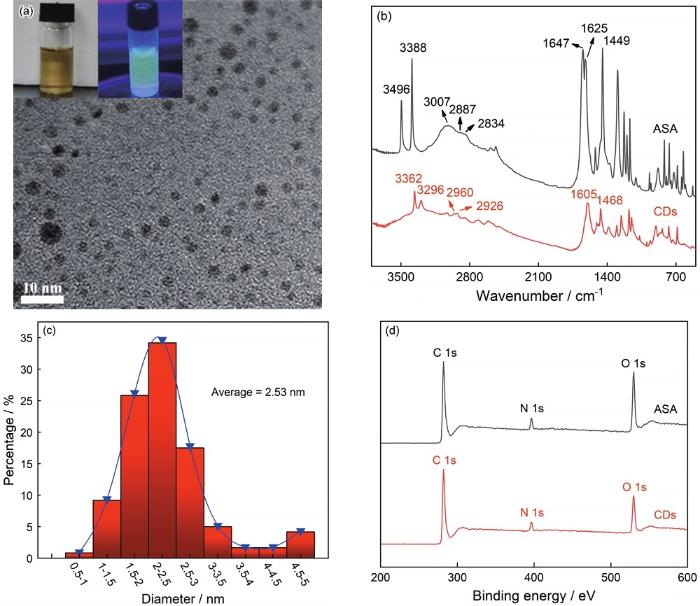
观察和统计结果表明,CDs的直径约为2.53 nm (图2a和c),CDs在365 nm的紫外线下显示出黄色荧光。分析ASA的FTIR光谱(图2b)发现,3496和3388 cm-1处的尖峰分别归属于O-H的拉伸振动和N-H拉伸振动[17]。3007~2834 cm-1之间的连续弱峰代表C-H键的拉伸振动 [17,18]。1647和1625 cm-1处的峰值归因于C=O振动和N-H弯曲 [19]。在1449和1295 cm-1处的尖峰是C-N和C-O基团的特征拉伸振动引起 [20]。而对于CDs, 未反应的O-H和N-H反映在3362和3296 cm-1处。由于分子分解、分子间环化和缩合反应等一系列反应,ASA中C=O的峰消失了。而在1605 cm-1处的一个新峰对应-NH2形变振动,1468 cm-1处的峰则与C=C振动有关。图2d中XPS谱图的元素分析则说明了ASA和CDs的组成元素相同,但是各元素的含量有所不同,且分子碳化现象明显(表1)。综上所述,ASA分子在反应过程中碳化明显,所含的一些基团已被分解,分子结构在一定程度上被破坏,生成了平均尺寸约为2.53 nm、具有荧光性的纳米粒子,即碳量子点。
图2
图2
CDs的TEM照片和在365 nm紫外光线下的荧光图像,CDs和ASA的红外光谱以及粒径分布和XPS能谱
Fig.2
TEM image of CDs and the photo pictures of the CDs solution in ethanol and the fluorescence image in 365 nm ultraviolet irradiation (a), FTIR spectra typical (b), particle size distribution of CDs (c) and typical XPS wide scans of ASA and CDs (d)
表1 ASA和CDs的元素含量
Table 1
| Sample | Atomic concentration (%, mole fraction) | ||
|---|---|---|---|
| O 1s | N 1s | C 1s | |
| ASA | 21.79 | 6.95 | 71.26 |
| CDs | 12.09 | 8.58 | 77.34 |
图3
图3
ASA和CDs的XPS元素精细谱图
Fig.3
XPS high resolution survey scans of (a, b) C 1s, and (c, d) O 1s regions of ASA and CDs
综上所述,ASA经过水热合成反应后分子结构遭到了破坏,在许多基团改变或消失的同时保留了很多预想中的特定结构,生成了尺寸小、基团丰富的碳量子点。
2.2 分离膜的表征
图4
图4
M0, M0.5, M5分离膜的显微红外谱图、XPS广谱以及不同组分分离膜的实物图
Fig.4
Micro-FTIR spectra (a) and typical XPS wide scans of M0, M0.5, M5 (b) and images of different membranes (c)
表2 不同组分分离膜的元素含量
Table 2
| Sample | Atomic concentration (%, mole fraction) | |||
|---|---|---|---|---|
| O 1s | N 1s | C 1s | S 2p | |
| M0 | 15.64 | - | 81.12 | 3.24 |
| M0.5 | 13.55 | 0.38 | 83.64 | 2.43 |
| M5 | 11.67 | 3.05 | 84.26 | 1.02 |
2.3 分离膜的微观形貌表征
图5
图5
分离膜表面的SEM照片
Fig.5
SEM images of the membranes (a) M0, (b) M0.5, (c) M1, (d) M2, (e) M3 and (f) M5
表3 分离膜表面的平均孔径和孔隙率
Table 3
| Simple | M0 | M0.5 | M1 | M2 | M3 | M5 |
|---|---|---|---|---|---|---|
| Average/nm | 9.4 ± 0.8 | 10.2 ± 0.9 | 13.6 ± 1.7 | 16.4 ± 2.4 | 20.1 ± 4.3 | 22.3 ± 8.8 |
| Porosity/% | 68.9 ± 0.5 | 69.6 ± 0.7 | 70.9 ± 0.6 | 73.6 ± 0.6 | 75.0 ± 0.9 | 77.3 ± 1.0 |
图6
图6
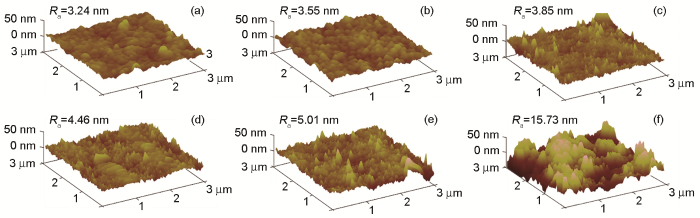
SPM表面粗糙度示意图
Fig.6
SPM images of the membranes (a) M0, (b) M0.5, (c) M1, (d) M2, (e) M3 and (f) M5
图7给出了分离膜的横截面微观结构。可以看出,随着CDs含量的提高指状结构变得更加明显,分离膜的内部变得中空且孔隙率增加。这些现象,可归因于CDs的致孔行为。在相转化成膜过程中,溶剂和致孔剂在自身亲水性的驱使下向着水相的方向移动并在膜中留下一条移动路径,即指状结构。随着CDs含量的提高分离膜内部的指状结构明显更加粗大,分离膜孔隙率上升即分离膜内部更加“空洞”,可能使膜分离效率和分离能力发生变化。
图7
图7
分离膜的截面结构SEM图像
Fig.7
Cross-section SEM images of M0 (a), M1 (b), M2 (c) and M5 (d)
2.4 分离膜的性能
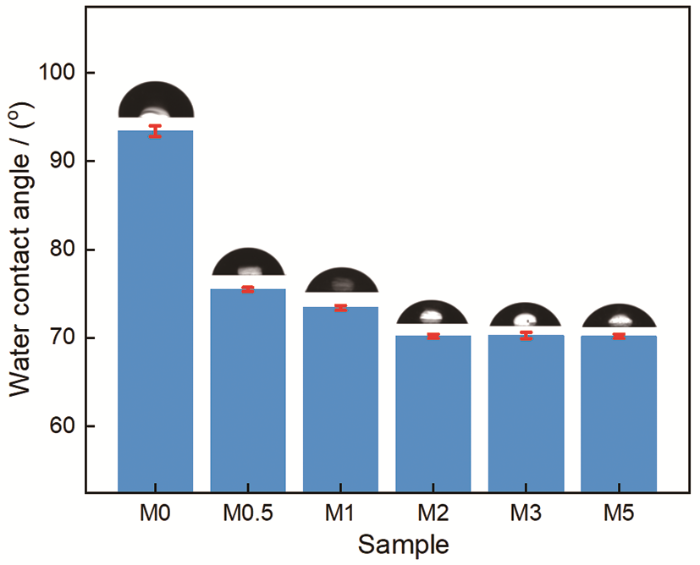
图8表明,纯PSF膜的水接触角为93.4°。随着CDs的引入,水接触角逐渐降低并稳定至70.2°。分离膜亲水性的提高,可归因于亲水性CDs基团的引入[23]。膜表面亲水基团的出现有助于在表面构筑水分子薄层,从而降低水和分离膜间的表面能改善分离膜表面的亲水性,因此水接触角呈现了明显下降的趋势。对于疏水性PSF膜,表面粗糙度的增加不利于亲水性的提高。基于分离膜表面微观结构的研究结果表明,CDs的引入使相转化过程更加快速和剧烈,因此分离膜的表面变得略微粗糙,但是CDs的添加量较低使分离膜表面所受影响有限。上述观点给出了CDs含量更高时反而接触角变化不大的原因,即分离膜改性不仅提高了亲水性,也略微增加了表面粗糙度,其结果是分离膜的水接触角趋于稳定。
图8
如图9a所示,纯水通量FW1从68.3(M0)增加到254.5 L·.m-2·h-1(M5)。其原因是,分离膜孔径和孔隙率的增大有利于水等液体的通过,即提高了分离膜的分离速率。同时,随着CDs含量的提高更多的CDs纳米粒子在相转化成膜过程中向铸膜液与水相间的界面运动,在即将凝固形成的分离膜内留下许多细微的孔洞。这些孔洞在分离液体时成为液体分子通过分离膜的路径,因而分离速率加快,表现为水通量的上升。
图9
图9
通量测试FW1, FBSA, FW2结果、BSA分子截留率RBSA及回复率FRR测试结果、总污染率(Rt)、可逆污染率(Rr)和不可逆污染率对比(Rir)以及可逆污染比(Rr/Rt)与不可逆污染比(Rir/Rt)的对比
Fig.9
The fluxes of FW1, FBSA, FW2 (a), BSA rejection and FRR of the fabricated membranes (b), total fouling ratio (Rt), reversible fouling ratios (Rr) and irreversible fouling ratios (Rir) (c), the percentage of reversible (Rr/Rt) and irreversible fouling (Rir/Rt) in total fouling of the different membranes (d)
分离膜的污染,主要是污染物的粘附和表面的强吸收或截留在膜的内部通道中引起的[23,24]。在过滤BSA溶液的过程中BSA分子因一些相互作用(例如与分离膜间的疏水作用、范德华力等)沉积在分离膜的表面阻塞了孔洞通道,随着分离开的BSA分子的累积在膜表面上形成滤饼层状物,使水分子更加难以通过即水通量降低。简单的震荡清洗即可清除并未吸附在膜表面的滤饼层,使水通量(FW2)有所恢复。但是,吸附在分离膜表面的BSA分子以及膜内部的分子则难以清除,使FW2明显小于FW1。而CDs的引入改善了分离膜表面的亲水性,不利于蛋白质类的疏水分子在膜表面吸附减少了污染。同时,污染物与分离膜间相互作用的减弱有利于降低清理污染时BSA分子从分离膜上脱离的难度。当CDs含量(质量分数)从0增至5%时清洗后的分离膜的回复水通量FW2明显提高,回复率FRR从67.6%增至91.6%,通量几乎达到了完全回复。这意味着,BSA分子类污染物经过简单的清洗过后即可被清除(图9b),说明本文制备的CDs/PSF共混膜具有比纯PSF膜更强的防污能力以及可重复使用性。
分离膜抗污染性提高的原因,还可从污染物类型的角度分析。根据分离膜污染类型及程度等因素,可将污染进行简单的划分:可用简单方法例如水清洗等即可轻松去除的污染为可逆污染,反之则是不可逆污染。针对图9c的污染类型进行总结,总污染率(Rt)从81.1%降低到了57.1%,表明改性后的分离膜的防污性有很大的提高。在图9d中,分离膜的不可逆污染与总污染之比(Rir/Rt)从40.0%(M0)降至14.7%(M5);可逆污染与总污染的比率(Rr/Rt)从60.1%(M0)增加到85.2%(M5),污染情况呈现下降且逐渐以可逆型污染为主体的趋势。其原因是,分离膜携带的亲水性基团有效地促进膜表面水层屏障的形成,使膜表面和污染物之间的粘附力变小。因此,很多污染物难于吸附在分离膜上,使污染由此前的不可逆型转变成易于去除的可逆型。当然,由于分离膜孔洞数量增多以及孔径的增大,较小的污染物更加易于通过分离膜,分离膜的分离能力下降,正如图9b中BSA分子截留率RBSA从81.5%(M0)降低到71.4%(M5)。因此,综合分离效率以及分离效果等多方面因素分析,2%(质量分数)的CDs含量是最佳比例,即M2分离膜具有较高的通量且其分离性能所受影响不大。
3 结论
(1) 用简单的水热合成法可制备具有一定亲水基团、平均粒径为2.53 nm的CDs,再用最简单的物理共混方法可制备CDs/PSF复合纳米分离膜。CDs可降低纳米材料在铸膜液中的团聚,减小对分离膜结构的破坏。这种复合分离膜具有更多的孔洞且表面略为粗糙,其内部有大量的孔洞。
(2) CDs类似于致孔剂在相转化过程中向着水相运动,从而在分离膜内产生更多更大的孔洞,可大幅提高分离膜的分离速率。
(3) CDs引入的亲水性基团提高了CDs/PSF复合膜的亲水性,能显著提高CDs/PSF复合膜的防污性能。M5的通量回复率FRR大于90%,总污染率Rt小于60%,而可逆污染比Rr/Rt可达85%以上。CDs含量为2%的分离膜,其综合分离效率、分离效果和防污能力等的整体效果最佳。
参考文献
Polysulfone ultrafiltration membranes impregnated with silver nanoparticles show improved biofouling resistance and virus removal
[J].Biofouling and virus penetration are two significant obstacles in water treatment membrane filtration. Biofouling reduces membrane permeability, increases energy costs, and decreases the lifetime of membranes. In order to effectively remove viruses, nanofiltration or reverse osmosis (both high energy filtration schemes) must be used. Thus, there is an urgent demand for low pressure membranes with anti-biofouling and antiviral properties. The antibacterial properties of silver are well known, and silver nanoparticles (nAg) are now incorporated into a wide variety of consumer products for microbial control. In this study, nAg incorporated into polysulfone ultrafiltration membranes (nAg-PSf) exhibited antimicrobial properties towards a variety of bacteria, including Escherichia coli K12 and Pseudomonas mendocina KR1, and the MS2 bacteriophage. Nanosilver incorporation also increased membrane hydrophilicity, reducing the potential for other types of membrane fouling. XPS analysis indicated a significant loss of silver from the membrane surface after a relatively short filtration period (0.4 L/cm2) even though ICP analysis of digested membrane material showed that 90% of the added silver remained in the membrane. This silver loss resulted in a significant loss of antibacterial and antiviral activity. Thus, successful fabrication of nAg-impregnated membranes needs to allow for the release of sufficient silver ions for microbial control while preventing a rapid depletion of silver.
Surface modification of polysulfone ultrafiltration membrane by oxygen plasma treatment
[J].
Chemically modified polysulfone hollow fibers with vinylpyrrolidone having improved blood compatibility
[J].
Characterization and performance studies of polysulfone membranes using PVP as an additive
[J].
Low temperature plasma treatment of asymmetric polysulfone membranes for permanent hydrophilic surface modification
[J].
Application of polysulfone (PSf)-and polyether ether ketone (PEEK)-tungstophosphoric acid (TPA) composite membranes for water electrolysis
[J].
Preparation and properties of functionalized carbon nanotube/PSF blend ultrafiltration membranes
[J].
Reduced wrinkling in GO membrane by grafting basal-plane groups for improved gas and liquid separations
[J].
Green synthesis of reduced graphene oxide/polyaniline composite and its application for salt rejection by polysulfone-based composite membranes
[J].
Carbon quantum dots and their applications
[J].Fluorescent carbon nanoparticles or carbon quantum dots (CQDs) are a new class of carbon nanomaterials that have emerged recently and have garnered much interest as potential competitors to conventional semiconductor quantum dots. In addition to their comparable optical properties, CQDs have the desired advantages of low toxicity, environmental friendliness low cost and simple synthetic routes. Moreover, surface passivation and functionalization of CQDs allow for the control of their physicochemical properties. Since their discovery, CQDs have found many applications in the fields of chemical sensing, biosensing, bioimaging, nanomedicine, photocatalysis and electrocatalysis. This article reviews the progress in the research and development of CQDs with an emphasis on their synthesis, functionalization and technical applications along with some discussion on challenges and perspectives in this exciting and promising field.
Carbon quantum dots/Ag3PO4 complex photocatalysts with enhanced photocatalytic activity and stability under visible light
[J].
Polyamine-functionalized carbon quantum dots for chemical sensing
[J].
Structural evolution of graphene quantum dots during thermal decomposition of citric acid and the corresponding photoluminescence
[J].
Synthesis of silver nanoparticles using reducing agents obtained from natural sources (Rumex hymenosepalus extracts)
[J].
Thin film nanocomposite membranes incorporated with graphene quantum dots for high flux and antifouling property
[J].
Topical treatment of distal ulcerative colitis with 4-amino-salicylic acid enemas
[J].Enemas of 4-amino-salicylic acid (4-ASA) have been compared with placebo enemas in a double-blind study for the treatment of distal ulcerative colitis of mild to moderate severity. 30 patients were randomised to receive enemas containing 1 g of 4-ASA or placebo, and 22 to receive 2-gram enemas of 4-ASA or placebo. For each dose of 4-ASA there was clinical and sigmoidoscopic improvement, which reached significance when all patients receiving 4-ASA enemas were compared with those receiving placebo enemas (Cochran's test: p less than 0.005). There was also a trend towards histological improvement, but this failed to reach the level of significance. These findings suggest that 4-ASA may provide a stable, inexpensive alternative to 5-ASA for the topical treatment of ulcerative colitis or for linking to carrier molecules for release in the colon.
Comparative randomized open study of the efficacy and tolerance of enemas with 2 gr of 4-amino-salicylic acid (4-ASA) and 1 gr of 5-amino-salicylic acid (5-ASA) in distal forms of hemorrhagic rectocolitis
[J].
Drug-derived bright and color-tunable N-doped carbon dots for cell imaging and sensitive detection of Fe(3+) in living cells
[J].
Preparation and properties of microporous membrane from poly (vinylidene fluoride-co-tetrafluoroethylene) (F2.4) for membrane distillation
[J].
Novel nitrogen doped carbon dots for corrosion inhibition of carbon steel in 1M HCl solution
[J].
Solvothermal synthesis of oxygen/nitrogen functionalized graphene-like materials with diversified morphology from different carbon sources and their fluorescence properties
[J].
Red, green, and blue luminescence by carbon dots: full-color emission tuning and multicolor cellular imaging
[J].
Enhanced hydrophilicity and salt rejection study of graphene oxide-polysulfone mixed matrix membrane
[J].
Organosilane-functionalized graphene oxide for enhanced antifouling and mechanical properties of polyvinylidene fluoride ultrafiltration membranes
[J].